
SL Paper 2
The following equation represents a combustion reaction of propane, \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{(g)}}\) when the oxygen supply is limited.
\[{{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{3CO(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\]
Define the term average bond enthalpy.
(i) Determine \(\Delta H\), the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using average bond enthalpy data from Table 10 of the Data Booklet. The bond enthalpy for the carbon-oxygen bond in carbon monoxide, CO, is \({\text{1072 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
(ii) The CO molecule has dative covalent bonding. Identify a nitrogen-containing positive ion which also has this type of bonding.
Markscheme
energy needed to break (1 mol of) a bond in a gaseous molecule/state/phase;
average calculated from a range of similar compounds / OWTTE;
Do not accept similar bonds instead of similar compounds.
M2 can be scored independently.
(i) Bonds breaking:
2 \( \times \) (C−C) + 8 \( \times \) (C−H) + 3.5 \( \times \) (O=O)
\( = (2)(347) + (8)(413) + (3.5)(498)\)
\( = {\text{5741(kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
3 \( \times \) (CO) + 8 \( \times \) (O−H)
\( = (3)(1072) + (8)(464) = 6928{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((5741 - 6928 = ) - 1187{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
(ii) \({\text{NH}}_{\text{4}}^ + \)/ammonium / \({{\text{N}}_{\text{2}}}{\text{H}}_5^ + \) /hydrazinium / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_3^ + \) /methylammonium / methanaminium / \({{\text{H}}_{\text{2}}}{\text{NO}}_3^ + \) /nitrooxonium;
Examiners report
The definition of average bond enthalpy in part (a), proved challenging even though it has appeared on recent examination papers and very few scored two marks. A good number of candidates omitted gaseous and did not state that it is the energy needed to break 1 mol of a bond in a gaseous molecule and many did not understand that it is the average calculated from a range of similar compounds.
In Part (b) (i), the typical errors were using the incorrect bond enthalpies from the Data Booklet and using the sum of the bond enthalpies of bond forming (products) minus bond breaking (reactants) instead of the reverse. In Part (b) (ii), instead of NH4+, candidates identified a range of incorrect answers including \({\text{NH}}_3^ + \), NF, \({\text{C}}{{\text{N}}^ - }\), \({\text{NO}}_3^ - \), N2 and even NaCl, although the question asked for a nitrogen containing positive ion.
Ethanol is used as a component in fuel for some vehicles. One fuel mixture contains 10% by mass of ethanol in unleaded petrol (gasoline). This mixture is often referred to as Gasohol E10.
Assume that the other 90% by mass of Gasohol E10 is octane. 1.00 kg of this fuel mixture was burned.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1367{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_8}{{\text{H}}_{18}}{\text{(l)}} + {\text{12}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{9}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 5470{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Calculate the mass, in g, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the amount, in mol, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the total amount of energy, in kJ, released when 1.00 kg of the fuel mixture is completely burned.
If the fuel blend was vaporized before combustion, predict whether the amount of energy released would be greater, less or the same. Explain your answer.
Markscheme
(10% 1000 g =) 100 g ethanol and (90% 1000 g =) 900 g octane;
\(n{\text{(ethanol)}} = 2.17{\text{ mol}}\) and \(n{\text{(octane)}} = 7.88{\text{ mol}}\);
\({{\text{E}}_{{\text{released from ethanol}}}} = (2.17 \times 1367) = 2966{\text{ (kJ)}}\);
\({{\text{E}}_{{\text{released from octane}}}} = (7.88 \times 5470) = 43104{\text{ (kJ)}}\);
total energy released \( = (2966 + 43104) = 4.61 \times {10^4}{\text{ (kJ)}}\);
Award [3] for correct final answer.
Accept answers using whole numbers for molar masses and rounding.
greater;
fewer intermolecular bonds/forces to break / vaporization is endothermic / gaseous fuel has greater enthalpy than liquid fuel / OWTTE;
M2 cannot be scored if M1 is incorrect.
Examiners report
Candidates were able to calculate the mass of ethanol and octane in the fuel mixture. The most common error here involved not expressing the answer in the requested units of grams. A number of candidates expressed answers in kg.
Many candidates were able to calculate the number of mole of ethanol and octane in (a) (ii) but errors in the calculation of molar mass were seen regularly. Candidates should also use the relative atomic masses, expressed to two decimal places as in the Periodic Table provided in the Data Table.
In part (a) (iii) some candidates multiplied incorrect numbers together or did not consider the number of moles of each part of the fuel mixture. Some candidates just added the enthalpies of combustion provided in the questions.
Part (b) was found to be very challenging by candidates. Very few candidates had the depth of understanding to answer this question adequately.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a base according to both the Brønsted–Lowry and the Lewis theories of acids and bases.
The equation for the reaction between sodium hydroxide, NaOH, and nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}\), is shown below.
\[\begin{array}{*{20}{l}} {{\text{NaOH(aq)}} + {\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaN}}{{\text{O}}_3}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}}&{{\text{ }}\Delta H = - 57.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Distinguish between the terms strong base and weak base, and state one example of each.
State the equation for the reaction of ammonia with water.
Explain why ammonia can act as a Brønsted–Lowry base.
Explain why ammonia can also act as a Lewis base.
(i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is added to excess solid sodium carbonate, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}{\text{(s)}}\), an acid–base reaction occurs. Bubbles of gas are produced and the solid sodium carbonate decreases in mass. State one difference which would be observed if nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), was used instead of ammonium chloride.
(ii) Deduce the Lewis structures of the ammonium ion, \({\text{NH}}_4^ + \), and the carbonate ion, \({\text{CO}}_3^{2 - }\).
Ammonium ion\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)Carbonate ion
(iii) Predict the shapes of \({\text{NH}}_4^ + \) and \({\text{CO}}_3^{2 - }\).
\({\text{NH}}_4^ + \):
\({\text{CO}}_3^{2 - }\):
(i) Sketch and label an enthalpy level diagram for this reaction.
(ii) Deduce whether the reactants or the products are more energetically stable, stating your reasoning.
(iii) Calculate the change in heat energy, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution is added to excess nitric acid.
When 5.35 g ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(s)}}\), is added to \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) of water, the temperature of the water decreases from 19.30 °C to 15.80 °C. Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the dissolving of ammonium chloride in water.
Markscheme
a strong base: base/electrolyte (assumed to be almost) completely/100% dissociated/ionized (in solution/water) / OWTTE and a weak base: base/electrolyte partially dissociated/ionized (in solution/water) / OWTTE;
example of a strong base: any group I hydroxide / \({\text{Ba(OH}}{{\text{)}}_2}\);
example of a weak base: \({\text{N}}{{\text{H}}_3}\) / \({\text{C}}{{\text{H}}_3}{\text{N}}{{\text{H}}_2}\) / any reasonable answer;
\({\text{N}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{NH}}_4^ + + {\text{O}}{{\text{H}}^ - }\);
accepts a proton/\({{\text{H}}^ + }\) / OWTTE;
donates an electron pair;
(i) more vigorous reaction / more gas bubbles / OWTTE;
more heat released;
solid decreases more quickly;
(ii) 
Accept any combination of lines, dots or crosses to represent electron pairs.
(iii) NH4+:
tetrahedral;
CO32–:
trigonal/triangular planar;
(i) enthalpy on y-axis;
Do not accept energy.
reactants higher than products;
\(\Delta H\) labelled;
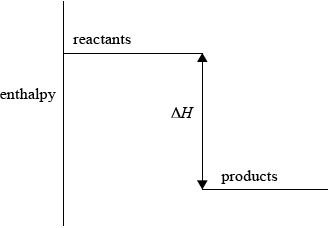
Accept appropriate formulas for reactants and products.
Arrow heads not needed.
57.6 is acceptable as an alternative to \(\Delta H\).
(ii) products are more stable as they are at a lower enthalpy level / energy has been given off by the reactants / reaction is exothermic / OWTTE;
(iii) \(n{\text{(NaOH)}} = 0.125{\text{ mol}}\);
change in heat energy \( = ( - 57.6 \times 0.125) = - 7.20{\text{ (kJ)}}\) / heat released \( = (57.6 \times 0.125) = 7.20{\text{ (kJ)}}\);
\(q = (mc\Delta T = ){\text{ }}100.0 \times 4.18 \times 3.50/1463{\text{ J}}/1460{\text{ J}}\);
\(n{\text{(N}}{{\text{H}}_{\text{4}}}{\text{Cl)}} = \frac{{5.35}}{{53.5}}/0.100{\text{ mol}}\);
\(\Delta H = + 14.6/14.6{\text{ (kJ mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept q = 105.35 \( \times \) 4.18 \( \times \) 3.50 / 1541 J.
Accept \(\Delta H\) = +15.4 / 15.4 (kJ\(\,\)mol–1)
Examiners report
Part (a) was answered well although some mentioned “dissolving” instead of “dissociating”.
In (b), the equation was well done.
In (b), the equation was well done as was (ii).
Inevitably, many omitted “pair” in (iii).
Part (c)(i) was generally correct. In (c)(ii) the carbonate ion was legitimately examined under AS 4.2.7; it was not well known – there were too many carbons with expanded octets and oxygens where the lone pairs had been missed. (In the HL specification, the carbonate ion‘s delocalization is considered.) In (iii), however, the shapes were well known.
If there was to be an error made in (d)(i), it was to omit “enthalpy” from the y-axis and some unaccountably put the correct chemicals on the line and then reversed the names products and reactants. The calculations in (d)(iii) inevitably depended on an ability to calculate and think logically.
The calculations in (e) inevitably depended on an ability to calculate and think logically.
Two students were asked to use information from the Data Booklet to calculate a value for the enthalpy of hydrogenation of ethene to form ethane.
\[{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
John used the average bond enthalpies from Table 10. Marit used the values of enthalpies of combustion from Table 12.
John then decided to determine the enthalpy of hydrogenation of cyclohexene to produce cyclohexane.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{10}}}}{\text{(l)}} + {{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{\text{(l)}}\]
Calculate the value for the enthalpy of hydrogenation of ethene obtained using the average bond enthalpies given in Table 10.
Marit arranged the values she found in Table 12 into an energy cycle.

Calculate the value for the enthalpy of hydrogenation of ethene from the energy cycle.
Suggest one reason why John’s answer is slightly less accurate than Marit’s answer.
Use the average bond enthalpies to deduce a value for the enthalpy of hydrogenation of cyclohexene.
The percentage difference between these two methods (average bond enthalpies and enthalpies of combustion) is greater for cyclohexene than it was for ethene. John’s hypothesis was that it would be the same. Determine why the use of average bond enthalpies is less accurate for the cyclohexene equation shown above, than it was for ethene. Deduce what extra information is needed to provide a more accurate answer.
Markscheme
energy required = C=C + H–H/612 + 436 and
energy released = C–C + 2(C–H)/347 + 2(413) /
energy required = C=C + H–H + 4(C–H)/612 + 436 + 4(413) and
energy released = C–C + 6(C–H)/347 + 6(413);
\(\Delta H = - 1411 + ( - 286) - ( - 1560) = - 137{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
the actual values for the specific bonds may be different to the average values / the combustion values referred to the specific compounds / OWTTE;
\( - {\text{125 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
average bond enthalpies do not apply to the liquid state / OWTTE;
the enthalpy of vaporization/condensation of cyclohexene and cyclohexane / OWTTE;
Examiners report
Candidates struggled with Part (a). The most common errors were those of calculation, incorrect identification of the bonds involved and a final answer with the opposite sign and missing units.
In (b) many candidates found it difficult to use Hess’ Law with the cycle presented in this form, a good proportion not recognising that this was, indeed, a Hess’ Law calculation.
In Part (c) many of the candidates simply repeated the question, giving no reason or explanation for the likely difference in accuracy.
Many candidates repeated the calculation from (a) in (d)(i) instead of realising that the question asked for a deduction rather than another calculation. Credit was given if the same (even if incorrect) answer was obtained as in part (a).
In (d)(ii) very few candidates seemed to notice that this process involved substances in the liquid state hence the need for enthalpies of vaporization/condensation. It was commonly thought that the position of the double bond in the cyclohexene ring would make a significant difference.
Define the term average bond enthalpy.
Deduce the balanced chemical equation for the complete combustion of butan-1-ol.
Determine the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the complete combustion of butan-1-ol, using the information from Table 10 of the Data Booklet.
Based on the types of intermolecular force present, explain why butan-1-ol has a higher boiling point than butanal.
Markscheme
energy required to break (1 mol of) a bond in a gaseous molecule/state;
Accept energy released when (1 mol of) a bond is formed in a gaseous molecule/state / enthalpy change when (1 mol of) bonds are formed or broken in the gaseous molecule/state.
average values obtained from a number of similar bonds/compounds / OWTTE;
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_3}{\text{OH(l)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}} \to {\text{4C}}{{\text{O}}_2}{\text{(g)}} + {\text{5}}{{\text{H}}_2}{\text{O(l)}}\);
Allow C4H9OH or C4H10O for CH3(CH2)3OH.
Ignore state symbols.
Bonds broken:
(6)(O=O) \( + \) (3)(C–C) \( + \) (1)(O–H) \( + \) (1)(C–O) \( + \) (9)(C–H) /
\(\left( {(6)(498) + (3)(347) + (1)(464) + (1)(358) + (9)(413) = } \right){\text{ }}8568{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds formed:
(8)(C=O) \( + \) (10)(O–H) / \(\left( {(8)(746) + (10)(464) = } \right){\text{ }}10608{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\Delta H{\text{ = }}(8568 - 10608 = ){\text{ }} - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +2040 (kJ\(\,\)mol–1).
hydrogen bonding in butan-1-ol;
stronger than dipole-dipole attractions in butanal;
Accept converse argument.
Do not penalize dipole-dipole bonding instead of dipole-dipole attractions.
Examiners report
Again this definition proved very challenging even though it has appeared on recent examination papers and very few scored both marks. Gaseous was often omitted and few stated that the average values are obtained from a number of similar bonds (again similar was often omitted).
In part (b) many of the better candidates were able to write the correct balanced combustion reaction. Some had an incorrect coefficient for oxygen and others wrote incorrect products which were often hydrocarbons.
In part (c) there were some fully correct responses, but many did lose marks. Common mistakes included using the O–O bond energy value instead of O=O. Others mixed up the signs.
In part (d) it was pleasing that nearly all candidates knew that hydrogen bonding occurs in butan-1-ol, but only the best students mentioned the dipole-dipole interactions in butanal. Generally butanal was described as having van der Waal’s or dispersion forces.
An example of a homogeneous reversible reaction is the reaction between hydrogen and iodine.
\[{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}\]
Propane can be formed by the hydrogenation of propene.
\[{\text{C}}{{\text{H}}_3}{\text{CH=C}}{{\text{H}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}}\]
Outline the characteristics of a homogeneous chemical system that is in a state of equilibrium.
Deduce the expression for the equilibrium constant, \({K_{\text{c}}}\).
Predict what would happen to the position of equilibrium and the value of \({K_{\text{c}}}\) if the pressure is increased from 1 atm to 2 atm.
The value of \({K_{\text{c}}}\) at 500 K is 160 and the value of \({K_{\text{c}}}\) at 700 K is 54. Deduce what this information tells us about the enthalpy change of the forward reaction.
The reaction can be catalysed by adding platinum metal. State and explain what effect the addition of platinum would have on the value of the equilibrium constant.
State the conditions necessary for the hydrogenation reaction to occur.
Enthalpy changes can be determined using average bond enthalpies. Define the term average bond enthalpy.
Determine a value for the hydrogenation of propene using information from Table 10 of the Data Booklet.
Explain why the enthalpy of hydrogenation of propene is an exothermic process.
Describe a chemical test that could be used to distinguish between propane and propene. In each case state the result of the test.
Under certain conditions propene can polymerize to form poly(propene). State the type of polymerization taking place and draw a section of the polymer to represent the repeating unit.
Other than polymerization, state one reaction of alkenes which is of economic importance.
Markscheme
reactants and products in same phase/state;
rate of forward reaction = rate of reverse reaction;
concentrations of reactants and products remain constant / macroscopic properties remain constant;
Do not accept concentrations are equal.
\(({K_{\text{c}}}) = \frac{{{{{\text{[HI]}}}^{\text{2}}}}}{{{\text{[}}{{\text{H}}_{\text{2}}}{\text{][}}{{\text{I}}_{\text{2}}}{\text{]}}}}\);
no change to position of equilibrium;
no change to value of \({K_{\text{c}}}\);
the reaction is exothermic/heat is given out/ \(\Delta H\) is negative;
no effect (on the value of the equilibrium constant);
as it speeds up forward and reverse reaction / concentrations of reactants and products do not change / position of equilibrium does not change / no change in yield;
nickel / platinum / paladium;
150 − 200 °C/ heat;
Accept temperatures in this range.
Accept room temperature as an answer if platinum or palladium used.
the enthalpy change when (one mole of) the gaseous bond is broken (or formed) / \({\text{X}}–{\text{Y(g)}} \to {\text{X(g)}} + {\text{Y(g)}}/{\text{X(g)}} + {\text{Y(g)}} \to {\text{X}}–{\text{Y(g)}}\);
averaged for the same bond in a number of similar compounds / OWTTE;
energy in: C=C + H–H and energy out: C–C + 2C–H;
Accept energy in C–C + 6C–H + C=C + H–H and energy out 2C–C + 8C–H.
\(\Delta H = (612 + 436) - (347 + 826) = 1048 - 1173/ - 125{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [2] for correct final answer.
Award [1] for +125.
If old Data Booklet values then allow: \(\Delta H = \) 1048−1172 = −124 (kJ\(\,\)mol–1)
due to the relative strength of the C–C and 2C–H bonds compared to the C=C and H–H bonds / bonds in products stronger than bonds in reactants;
(i) addition of bromine/bromine water;
the bromine colour remains with propane and propene decolourizes the bromine / solution changes from brown to colourless;
Do not accept “clear” instead of “colourless”.
addition (polymerization);
−(−CH(\({\text{C}}{{\text{H}}_{\text{3}}}\))−\({\text{C}}{{\text{H}}_{\text{2}}}\)−)− / −CH(\({\text{C}}{{\text{H}}_{\text{3}}}\))CH−;
Continuation bonds necessary for mark, displayed formula or condensed structural formula can be given.
Accept if more than one repeating unit is shown.
hydrogenation (of vegetable oils) / manufacture of margarine / manufacture of ethanol / addition of water;
Accept manufacture of alcohol.
Do not accept hydrogenation of alkenes.
Examiners report
Part (a) of this question focused on equilibrium and many candidates were able to show a good understanding of what would happen when the conditions were changed and were able to deduce the equilibrium expression. Most could describe the properties of a homogeneous equilibrium but some said that concentrations of reactants and products were equal at equilibrium as opposed to constant. The candidates also could state and explain the effect of a catalyst.
Part (a) of this question focused on equilibrium and many candidates were able to show a good understanding of what would happen when the conditions were changed and were able to deduce the equilibrium expression. Most could describe the properties of a homogeneous equilibrium but some said that concentrations of reactants and products were equal at equilibrium as opposed to constant. The candidates also could state and explain the effect of a catalyst.
Part (a) of this question focused on equilibrium and many candidates were able to show a good understanding of what would happen when the conditions were changed and were able to deduce the equilibrium expression. Most could describe the properties of a homogeneous equilibrium but some said that concentrations of reactants and products were equal at equilibrium as opposed to constant. The candidates also could state and explain the effect of a catalyst.
Part (a) of this question focused on equilibrium and many candidates were able to show a good understanding of what would happen when the conditions were changed and were able to deduce the equilibrium expression. Most could describe the properties of a homogeneous equilibrium but some said that concentrations of reactants and products were equal at equilibrium as opposed to constant. The candidates also could state and explain the effect of a catalyst.
Part (a) of this question focused on equilibrium and many candidates were able to show a good understanding of what would happen when the conditions were changed and were able to deduce the equilibrium expression. Most could describe the properties of a homogeneous equilibrium but some said that concentrations of reactants and products were equal at equilibrium as opposed to constant. The candidates also could state and explain the effect of a catalyst.
Part (b) proved more problematic and relatively few could describe the necessary conditions for hydrogenation, and even fewer could correctly state a definition of average bond enthalpy. The calculation of the bond enthalpy of propene proved difficult for many and although some gained marks by ecf few obtained the correct answer -125. Candidates also had difficulty explaining why the process was exothermic in terms of the relative strengths of the bonds being made and broken.
Part (b) proved more problematic and relatively few could describe the necessary conditions for hydrogenation, and even fewer could correctly state a definition of average bond enthalpy. The calculation of the bond enthalpy of propene proved difficult for many and although some gained marks by ecf few obtained the correct answer -125. Candidates also had difficulty explaining why the process was exothermic in terms of the relative strengths of the bonds being made and broken.
Part (b) proved more problematic and relatively few could describe the necessary conditions for hydrogenation, and even fewer could correctly state a definition of average bond enthalpy. The calculation of the bond enthalpy of propene proved difficult for many and although some gained marks by ecf few obtained the correct answer -125. Candidates also had difficulty explaining why the process was exothermic in terms of the relative strengths of the bonds being made and broken.
Part (b) proved more problematic and relatively few could describe the necessary conditions for hydrogenation, and even fewer could correctly state a definition of average bond enthalpy. The calculation of the bond enthalpy of propene proved difficult for many and although some gained marks by ecf few obtained the correct answer -125. Candidates also had difficulty explaining why the process was exothermic in terms of the relative strengths of the bonds being made and broken.
Part (c) was also based in organic chemistry and although most candidates could suggest bromine as a test for unsaturation, they did not all state a correct test result.
Candidates must make sure that they state that the bromine becomes colourless and not clear. Many realised that propene polymerises by addition polymerisation but few could successfully draw the structure of the repeating unit. Also few could suggest a reaction of alkenes of economic importance- such as hydration to make alcohols.
Part (c) was also based in organic chemistry and although most candidates could suggest bromine as a test for unsaturation, they did not all state a correct test result.
Candidates must make sure that they state that the bromine becomes colourless and not clear. Many realised that propene polymerises by addition polymerisation but few could successfully draw the structure of the repeating unit. Also few could suggest a reaction of alkenes of economic importance- such as hydration to make alcohols.
Part (c) was also based in organic chemistry and although most candidates could suggest bromine as a test for unsaturation, they did not all state a correct test result.
Candidates must make sure that they state that the bromine becomes colourless and not clear. Many realised that propene polymerises by addition polymerisation but few could successfully draw the structure of the repeating unit. Also few could suggest a reaction of alkenes of economic importance- such as hydration to make alcohols.
Enthalpy changes depend on the number and type of bonds broken and formed.
The table lists the standard enthalpies of formation, \(\Delta H_{\text{f}}^\Theta \), for some of the species in the reaction above.
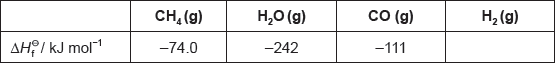
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
Outline why no value is listed for H2(g).
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
Outline why the value of enthalpy of reaction calculated from bond enthalpies is less accurate.
Markscheme
bonds broken: 4(C–H) + 2(H–O)/4(414) + 2(463)/2582 «kJ»
bonds made: 3(H–H) + C≡O/3(436) + 1077/2385 «kJ»
ΔH «= ΣBE(bonds broken) – ΣBE(bonds made) = 2582 – 2385» = «+» 197 «kJ»
Award [3] for correct final answer.
Award [2 max] for –197 «kJ».
[3 marks]
\(\Delta H_{\text{f}}^\Theta \) for any element = 0 «by definition»
OR
no energy required to form an element «in its stable form» from itself
[1 mark]
ΔHΘ « = \(\sum {\Delta H_{\text{f}}^\Theta } \)(products) – \(\sum {\Delta H_{\text{f}}^\Theta } \)(reactants) = –111 + 0 – [–74.0 + (–242)]»
= «+» 205 «kJ»
[1 mark]
«bond enthalpies» averaged values «over similar compounds»
OR
«bond enthalpies» are not specific to these compounds
[1 mark]
Examiners report
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) \( \rightleftharpoons \) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Markscheme
(i)
\(\ll {K_{\text{C}}} = \gg \frac{{\left[ {{\text{HOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}} \right]}}{{{{\left[ {{\text{CO}}} \right]}^{\text{2}}} \times {{\left[ {{{\text{H}}_{\text{2}}}} \right]}^{\text{3}}}}}\)
(ii)
Position of equilibrium: moves to right OR favours product
Kc: no change OR is a constant at constant temperature
(iii)
Bonds broken: 2C≡O + 3(H-H) / 2(1077kJmol-1) + 3(436kJmol-1) / 3462 «kJ»
Bonds formed: 2(C-O) + 2(O-H) + 4(C-H) + (C-C) / 2(358kJmol-1) + 2(463kJmol-1) + 4(414kJmol-1) + 346kJmol-1 / 3644 «kJ»
«Enthalpy change = bonds broken - bonds formed = 3462 kJ - 3644 kJ =» -182 «kJ»
Award [3] for correct final answer.
Award [2 max] for «+»182 «kJ».
(iv)
in (a)(iii) gas is formed and in (a)(iv) liquid is formed
OR
products are in different states
OR
conversion of gas to liquid is exothermic
OR
conversion of liquid to gas is endothermic
OR
enthalpy of vapourisation needs to be taken into account
Accept product is «now» a liquid.
Accept answers referring to bond enthalpies being means/averages.
Ethene: –2
Ethane-1,2-diol: –1
Do not accept 2–, 1– respectively.
ethane-1,2-diol can hydrogen bond to other molecules «and ethene cannot»
OR
ethane-1,2-diol has «significantly» greater van der Waals forces
Accept converse arguments.
Award [0] if answer implies covalent bonds are broken
hydrogen bonding is «significantly» stronger than other intermolecular forces
acidified «potassium» dichromate«(VI)»/H+ AND K2Cr2O7/H+ AND Cr2O72-
OR
«acidified potassium» manganate(VII)/ «H+» KMnO4 /«H+» MnO4-
Accept Accept H2SO4 or H3PO4 for H+.
Accept “permanganate” for “manganate(VII)”.
Examiners report
If white anhydrous copper(II) sulfate powder is left in the atmosphere it slowly absorbs water vapour giving the blue pentahydrated solid.
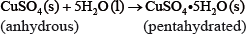
It is difficult to measure the enthalpy change for this reaction directly. However, it is possible to measure the heat changes directly when both anhydrous and pentahydrated copper(II) sulfate are separately dissolved in water, and then use an energy cycle to determine the required enthalpy change value, \(\Delta {H_{\text{x}}}\), indirectly.
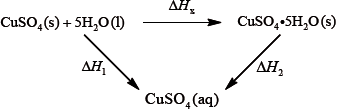
To determine \(\Delta {H_1}\) a student placed 50.0 g of water in a cup made of expanded polystyrene and used a data logger to measure the temperature. After two minutes she dissolved 3.99 g of anhydrous copper(II) sulfate in the water and continued to record the temperature while continuously stirring. She obtained the following results.
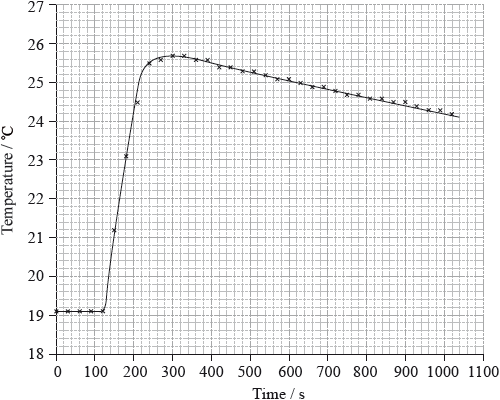
To determine \(\Delta {H_2}\), 6.24 g of pentahydrated copper(II) sulfate was dissolved in 47.75 g of water. It was observed that the temperature of the solution decreased by 1.10 °C.
The magnitude (the value without the \( + \) or \( - \) sign) found in a data book for \(\Delta {H_{\text{x}}}\) is \({\text{78.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of anhydrous copper(II) sulfate dissolved in the 50.0 g of water.
Determine what the temperature rise would have been, in °C, if no heat had been lost to the surroundings.
Calculate the heat change, in kJ, when 3.99 g of anhydrous copper(II) sulfate is dissolved in the water.
Determine the value of \(\Delta {H_1}{\text{ in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of water in 6.24 g of pentahydrated copper(II) sulfate.
Determine the value of \(\Delta {H_2}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Using the values obtained for \(\Delta {H_1}\) in (a) (iv) and \(\Delta {H_2}\) in (b) (ii), determine the value for \(\Delta {H_{\text{x}}}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the percentage error obtained in this experiment. (If you did not obtain an answer for the experimental value of \(\Delta {H_{\text{x}}}\) then use the value \({\text{70.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the true value.)
The student recorded in her qualitative data that the anhydrous copper(II) sulfate she used was pale blue rather than completely white. Suggest a reason why it might have had this pale blue colour and deduce how this would have affected the value she obtained for \(\Delta {H_{\text{x}}}\).
Markscheme
\({\text{amount}} = \frac{{3.99}}{{159.61}} = 0.0250{\text{ (mol)}}\);
26.1 (°C);
Accept answers between 26.0 and 26.2 ( °C).
temperature rise \( = 26.1 - 19.1 = 7.0\) (°C);
Accept answers between 6.9 °C and (7.1 °C) .
Award [2] for the correct final answer.
No ECF if both initial and final temperatures incorrect.
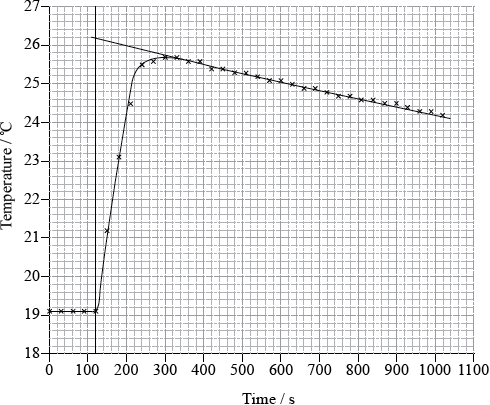
heat change \( = \frac{{50.0}}{{1000}} \times 4.18 \times 7.0/50.0 \times 4.18 \times 7.0\);
Accept 53.99 instead of 50.0 for mass.
\( = 1.5{\text{ (kJ)}}\);
Allow 1.6 (kJ) if mass of 53.99 is used.
Ignore sign.
\(\Delta {H_1} = \frac{{1.5}}{{0.0250}} = - 60{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Value must be negative to award mark.
Accept answers in range –58.0 to –60.0.
Allow –63 (kJ\(\,\)mol–1) if 53.99 g is used in (iii).
\({\text{(amount of CuS}}{{\text{O}}_4} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}} = \frac{{6.24}}{{249.71}} = {\text{) 0.0250 (mol)}}\);
\({\text{(amount of }}{{\text{H}}_{\text{2}}}{\text{O in 0.0250 mol of CuS}}{{\text{O}}_{\text{4}}} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}} = 5 \times 0.0250 = {\text{) 0.125 (mol)}}\).
\((50.0 \times 4.18 \times 1.10 = ){\text{ }}230{\text{ (J)}}\);
\(\left( {\frac{{229.9}}{{(1000{\text{ }}0.0250)}} = } \right){\text{ }} + 9.20{\text{ (kJ)}}\);
Accept mass of 47.75 or 53.99 instead of 50.00 giving answers of +.8.78 or +9.9.
Do not penalize missing + sign but penalize – sign unless charge already penalized in (a) (iv).
\(\left( {\Delta {H_{\text{x}}} = \Delta {H_2} - \Delta {H_2} = - 58.4 - ( + 9.20) = } \right){\text{ }} - 67.6{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\)
\(\frac{{[ - 78.0 - ( - 67.6)]}}{{ - 78.0}} \times 100 = 13.3\% \);
If 70.0 kJ\(\,\)mol−1 is used accept 10.3%.
the anhydrous copper(II) sulfate had already absorbed some water from the air / OWTTE;
the value would be less exothermic/less negative than expected as the temperature increase would be lower / less heat will be evolved when the anhydrous salt is dissolved in water / OWTTE;
Do not accept less without a reason.
Examiners report
Question 1 was a generally difficult question for candidates, but most students did pick up marks thanks to the application of error carried forward (ecf). In part (a) students could usually calculate the moles of anhydrous copper sulphate.
Very few candidates could correctly extrapolate the graph to calculate a temperature rise of 7.0 ºC.
Calculating using \({\text{q}} = {\text{mc}}\Delta {\text{T}}\) also caused problems as many students used the mass of the copper sulphate instead of the mass of water, and some also added 273 to the temperature change. Many candidates also forgot to convert to kJ.
The last part of this question required the calculation of \(\Delta H\), here many students forgot the – symbol to indicate it was exothermic and so did not gain the mark.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (c) many could calculate the % error and apply Hess’s law to calculate \(\Delta H\). Throughout this question there were numerous instances of students using an incorrect number of significant figures and this led to another mark being lost.
Consider the following equilibrium:
\[\begin{array}{*{20}{l}} {{\text{4N}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{4NO(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(g)}}}&{\Delta {H^\Theta } = - 909{\text{ kJ}}} \end{array}\]
Nitrogen reacts with hydrogen to form ammonia in the Haber process, according to the following equilibrium.
\[\begin{array}{*{20}{l}} {{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 92.6{\text{ kJ}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict the direction in which the equilibrium will shift when the following changes occur.
The volume increases.
The temperature decreases.
\({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is removed from the system.
A catalyst is added to the reaction mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Nitrogen monoxide, NO, is involved in the decomposition of ozone according to the following mechanism.
\[\begin{array}{*{20}{l}} {}&{{{\text{O}}_{\text{3}}} \to {{\text{O}}_{\text{2}}} + {\text{O}} \bullet } \\ {}&{{{\text{O}}_3} + {\text{NO}} \to {\text{N}}{{\text{O}}_2} + {{\text{O}}_2}} \\ {}&{{\text{N}}{{\text{O}}_2} + {\text{O}} \bullet \to {\text{NO}} + {{\text{O}}_2}} \\ {{\text{Overall:}}}&{{\text{2}}{{\text{O}}_3} \to {\text{3}}{{\text{O}}_2}} \end{array}\]
State and explain whether or not NO is acting as a catalyst.
Define the term endothermic reaction.
Sketch the Maxwell-Boltzmann energy distribution curve for a reaction with and without a catalyst, and label both axes.
Define the term rate of reaction.
Iron, used as the catalyst in the Haber process, has a specific heat capacity of \({\text{0.4490 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). If 245.0 kJ of heat is supplied to 8.500 kg of iron, initially at a temperature of 15.25 °C, determine its final temperature in K.
Markscheme
\({\text{(}}{K_{\text{c}}}{\text{)}} = \frac{{{{{\text{[NO]}}}^4}{{{\text{[}}{{\text{H}}_2}{\text{O]}}}^6}}}{{{{{\text{[N}}{{\text{H}}_3}{\text{]}}}^4}{{{\text{[}}{{\text{O}}_2}{\text{]}}}^5}}}\);
No mark if square brackets are omitted or are incorrect.
right;
right;
right;
no change;
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Allow energy difference between reactants and transition state.
catalyst;
regenerated at end of reaction / OWTTE;
(system) absorbs/takes in heat from surroundings / OWTTE;
Allow standard enthalpy change/ \(\Delta {H^\Theta }\) positive.
Allow bond breaking more energetic then bond formation / OWTTE.
Absorbs/takes in heat alone not sufficient for mark.
Curve showing:
general shape of Maxwell-Boltzmann energy distribution curve;
correct position of \({E_{\text{a}}}\) (catalysed) and \({E_{\text{a}}}\) (uncatalysed);
labelled y-axis: probability of particles (with that kinetic energy) and labelled x-axis: (kinetic) energy;
Allow number/fraction/proportion of particles (with kinetic energy) for y-axis label, but do not allow amount or particles.
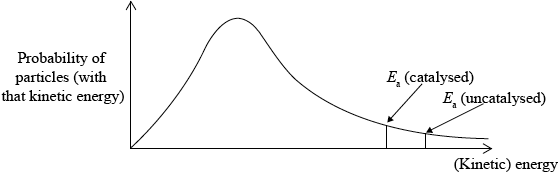
Award [2 max] if a second curve is drawn, but at a higher temperature, M2 will not be scored here.
change in concentration of reactant/product with time / rate of change of concentration;
Increase can be used instead of change for product or decrease can be used instead of change for reactant.
Allow mass/amount/volume instead of concentration.
Do not accept substance.
\(q = mc\Delta T = 2.450 \times {10^5} = (8.500 \times {10^3})(0.4490)({T_{\text{f}}} - 15.25)\);
\({T_{\text{f}}} = 79.44{\text{ °C}}/\Delta T = 64.19{\text{ (°C}}/{\text{K)}}\);
\({T_{\text{f}}} = (79.44 + 273) = 352{\text{ (K)}}\);
Award [3] for correct final answer.
Accept the use of 273.15 K instead of 273 K giving final value of 352.59 K.
For M1 and M2 award [1 max] for use of \(q = mc\Delta T\) if incorrect units of m and c are used.
Examiners report
In part (a) of this question the \({K_{\text{c}}}\) expression was usually written correctly though the very weak students did mix up the numerator and denominator in (i), or include a + sign between substances.
Candidates generally had few problems, but the reaction condition that proved to be the most the most difficult factor was the volume.
Activation energy was often clearly defined though some forgot to mention minimum.
The best students realised that NO acted as a catalyst as it was regenerated at the end of the reaction. However many weaker students stated it was not a catalyst as it was not involved in the reaction.
The definition of an endothermic reaction was generally well answered, however some just said it absorbs heat and forgot to mention the surroundings in their answer.
Incorrect labels for the axes were often seen, as well as a very high proportion of symmetrical curves, some which did not start at the origin. Also many drew two curves. Also in some cases the catalyzed and uncatalyzed activation energies were often mixed up. The weaker students drew an enthalpy level diagram instead of a Maxwell-Boltzmann distribution.
In the definition for rate of reaction some students forgot to mention concentration.
This question is about ethene, C2H4, and ethyne, C2H2.
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHϴ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
Explain, giving two reasons, the difference in the values for (b)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
One possible Lewis structure for benzene is shown.
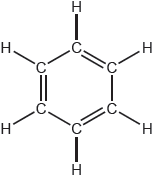
State one piece of physical evidence that this structure is incorrect.
State the characteristic reaction mechanism of benzene.
Markscheme
nickel/Ni «catalyst»
high pressure
OR
heat
Accept these other catalysts: Pt, Pd, Ir, Rh, Co, Ti.
Accept “high temperature” or a stated temperature such as “150 °C”.
[2 marks]
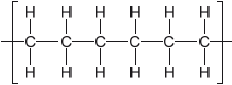
Ignore square brackets and “n”.
Connecting line at end of carbons must be shown.
[1 mark]
ΔHϴ = bonds broken – bonds formed
«ΔHϴ = 3(C≡C) – 6(C=C)benzene/3 × 839 – 6 × 507 / 2517 – 3042 =»
–525 «kJ»
Award [2] for correct final answer.
Award [1 max] for +525 «kJ»
Award [1 max] for:
«ΔHϴ = 3(C≡C) – 3(C–C) – 3(C=C) / 3 × 839 – 3 × 346 – 3 × 614 / 2517 – 2880 =» –363 «kJ».
[2 marks]
ΔHΘ = ΣΔHf(products) – ΣΔHf(reactants)
«ΔHΘ = 49 kJ – 3 × 228 kJ =» –635 «kJ»
Award [2] for correct final answer.
Award [1 max] for “+635 «kJ»”.
[2 marks]
ΔHf values are specific to the compound
OR
bond enthalpy values are averages «from many different compounds»
condensation from gas to liquid is exothermic
Accept “benzene is in two different states «one liquid the other gas»“ for M2.
[2 marks]
equal C–C bond «lengths/strengths»
OR
regular hexagon
OR
«all» C–C have» bond order of 1.5
OR
«all» C–C intermediate between single and double bonds
Accept “all C–C–C bond angles are equal”.
[1 mark]
electrophilic substitution
OR
SE
[1 mark]
Examiners report
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated below.
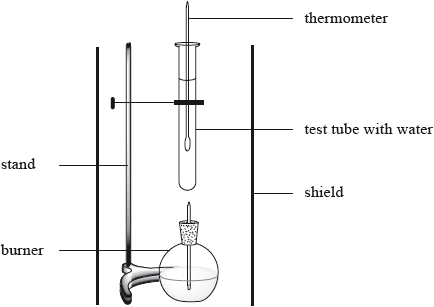
The following data were collected.

The Data Booklet value for the enthalpy of combustion of methanol is \( - {\text{726 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Suggest why this value differs from the values calculated in parts (a) and (b).
Using the information from Table 10 of the Data Booklet, determine the theoretical enthalpy of combustion of methanol.
Calculate the amount, in mol, of methanol burned.
Calculate the heat absorbed, in kJ, by the water.
Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the combustion of 1 mole of methanol.
Part (a)
Part (b)
Markscheme
amount of energy required to break bonds of reactants
\(3 \times 413 + 358 + 464 + 1.5 \times 498{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/2808{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
amount of energy released during bond formation of products
\(4 \times 464 + 2 \times 746{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/3348{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\Delta H = - 540{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for (+)540.
If old Data Booklet is used accept answer: –535 (kJ\(\,\)mol–1) or award [2] for (+)535.
\(m{\text{(methanol)}} = (80.557 - 80.034) = 0.523{\text{ (g)}}\);
\(n{\text{(methanol)}} = \left( {\frac{{0.523{\text{ g}}}}{{32.05{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}} \right) = 0.0163{\text{ (mol)}}\);
Award [2] for correct final answer.
\(\Delta T = (26.4 - 21.5) = 4.9{\text{ (K)}}\);
\(q = (mc\Delta T = ){\text{ }}20.000 \times 4.18 \times 4.9{\text{ (J)}}/20.000 \times 4.18 \times 4.9 \times {10^{ - 3}}{\text{ (kJ)}}\);
0.41 (kJ);
Award [3] for correct final answer.
\(\Delta H_{\text{c}}^\Theta = - \frac{{0.41{\text{ (kJ)}}}}{{0.0163{\text{ (mol)}}}}/ - 25153{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\( = - 25{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [2] for correct final answer.
Award [1] for (+)25 (kJ\(\,\)mol–1).
bond enthalpies are average values/differ (slightly) from one compound to another (depending on the neighbouring atoms) / methanol is liquid not gas in the reaction;
not all heat produced transferred to water / heat lost to surroundings/environment / OWTTE / incomplete combustion (of methanol) / water forms as \({\text{ }}{{\text{H}}_2}{\text{O(l)}}\) instead of \({{\text{H}}_2}{\text{O(g)}}\);
Do not allow just “heat lost”.
Examiners report
Many errors were seen in part (a). Candidates used the wrong values from the Data Booklet, wrong coefficients were used and not all the correct bonds were selected. Some candidates also reversed the final calculation to get an endothermic enthalpy rather than an exothermic enthalpy or made careless arithmetic errors.
Candidates were proficient at correctly calculating the number of mole methanol burnt.
Candidates did not use the expression \(q = mc\Delta T\) well.
Again numerous errors were seen here with candidates using the mass of methanol rather than water, adding 273 to the temperature change calculated and not converting J to kJ. Some candidates did not recognise that the combustion of methanol is exothermic and hence did not include the negative sign for the enthalpy change.
Part (c) was generally well done, however candidates often just stated that ‘heat was lost’ in part (ii). A more detail response was expected, e.g. heat was lost to surroundings.
Part (c) was generally well done, however candidates often just stated that ‘heat was lost’ in part (ii). A more detail response was expected, e.g. heat was lost to surroundings.
In December 2010, researchers in Sweden announced the synthesis of N,N–dinitronitramide, \({\text{N(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{3}}}\). They speculated that this compound, more commonly called trinitramide, may have significant potential as an environmentally friendly rocket fuel oxidant.
Methanol reacts with trinitramide to form nitrogen, carbon dioxide and water. Deduce the coefficients required to balance the equation for this reaction.
___ \({\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \) ___ \({\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \) ___ \({{\text{N}}_2}{\text{(g)}} + \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}} + \) ___ \({{\text{H}}_2}{\text{O(l)}}\)
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), when one mole of trinitramide decomposes to its elements, using bond enthalpy data from Table 10 of the Data Booklet. Assume that all the N–O bonds in this molecule have a bond enthalpy of \({\text{305 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Outline how the length of the N–N bond in trinitramide compares with the N–N bond in nitrogen gas, \({{\text{N}}_{\text{2}}}\).
Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
Predict, with an explanation, the polarity of the trinitramide molecule.
Methanol can also be burnt as a fuel. Describe an experiment that would allow the molar enthalpy change of combustion to be calculated from the results.
Explain how the results of this experiment could be used to calculate the molar enthalpy change of combustion of methanol.
Predict, with an explanation, how the result obtained would compare with the value in Table 12 of the Data Booklet.
Markscheme
\(\underline {{\text{ (1) }}} {\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \underline {{\text{ 2 }}} {\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \underline {{\text{ 2 }}} {{\text{N}}_2}{\text{(g)}} + \underline {{\text{ 2 }}} {\text{C}}{{\text{O}}_2}{\text{(g)}} + \underline {{\text{ 4 }}} {{\text{H}}_2}{\text{O(l)}}\);
bonds broken: \((6 \times 305) + (3 \times 158) = 1830 + 474 = 2304{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
bonds made: \((2 \times 945) + (3 \times 498) = 1890 + 1494 = 3384{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
enthalpy change: \(2304 - 3384 = - 1080{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Award [2 max] for +1080 (kJ mol–1) .
Accept –234 kJ mol–1 which arise from students assuming that 305 kJ mol–1 refers to the strength of a single N–O bond. Students may then take N=O from the data book value (587 kJ mol–1).
bonds broken: (3 \( \times \) 305) + (3 \( \times \) 587) + (3 \( \times \) 158) = 915 + 1761 + 474 = 3150 (kJ mol–1)
bonds made: (2 \( \times \) 945) + (3 \( \times \) 498) = 1890 + 1494 = 3384 (kJ mol–1)
enthalpy change: 3150 – 3384 = –234 (kJ mol–1) .
Award [2 max] for correct calculation of the enthalpy change of reaction for the equation in part (a), which gives –2160 (kJ mol–1).
Award [1] if the final answer is not –2160 but the candidate has correctly calculated the bonds broken in trinitramide as 2304 (kJ mol–1).
(N–N bond in) trinitramide is longer/nitrogen (gas) is shorter / 0.145 nm in trinitramide versus 0.110 nm in nitrogen;
trinitramide has single (N–N) bond and nitrogen (gas) has triple bond;
106°–108°;
Accept \( < \)109°.
Any two for [2 max].
4 (negative) charge centres/electron pairs/electron domains around central nitrogen;
central nitrogen has a lone/non-bonding pair;
lone/non-bonding pairs repel more than bonding pairs;
molecule will be (trigonal/triangular) pyramidal;
(negative) charge centres/electron pairs/electron domains will be tetrahedrally arranged/orientated/ have tetrahedral geometry;
Do not apply ECF.
polar;
net dipole moment present in molecule / unsymmetrical distribution of charge / polar bonds do not cancel out / centre of negatively charged oxygen atoms does not coincide with positively charged nitrogen atom;
Marks may also be awarded for a suitably presented diagram showing net dipole moment.
Do not accept “unsymmetrical molecule”.
For polarity, apply ECF from part (e).
burn/combust a (known) mass/volume/quantity/amount of methanol (in a spirit burner) / weigh methanol/spirit burner before and after combustion;
use flame to heat a (known) mass/volume/quantity/amount of water;
measure the increase/rise/change in temperature (of the water);
calculate the heat gained by the water / calculate the heat evolved by the burning methanol / substitute in \(q = mc\Delta T\);
calculate the amount/moles of methanol / divide the mass of methanol by its molar mass;
divide the heat gained by the water by the amount/moles of methanol;
result would be less exothermic/less negative;
Accept “less/smaller/lower”.
heat loss / incomplete combustion;
Accept methanol is volatile/evaporates / beaker/material of calorimeter absorbs heat.
Examiners report
Most candidates got the correct stoichiometric coefficients for the equation in part (a).
In Part (c), the typical errors were using the incorrect bond enthalpies from the Data Booklet and using the sum of the bond enthalpies of bond forming (products) minus bond breaking (reactants) instead of the reverse. Some candidates surprisingly used the combustion equation from part (a) for their extensive calculations which was partially given credit.
Part (d) was well answered although a number of candidates thought that nitrogen has a single or double bond instead of a triple bond which was worrying. VSEPR theory however was exceptionally poor and most candidates demonstrated little or no understanding. Many incorrect geometries were cited, especially trigonal planar and even linear and v-shaped! Very few candidates related the geometry to four negative charge centres or electron domains around the central nitrogen atom.
In part (f), polarity typically involved just guess work and only few candidates could explain the reason for the polarity or gave a diagram showing the net dipole moment which suggested poor understanding of the topic.
Part (g) was generally well answered and of those that attempted the question they often scored full marks demonstrating good understanding of calorimetry.
Part (g) was generally well answered and of those that attempted the question they often scored full marks demonstrating good understanding of calorimetry.
Part (g) was generally well answered and of those that attempted the question they often scored full marks demonstrating good understanding of calorimetry.
In some countries, ethanol is mixed with gasoline (petrol) to produce a fuel for cars called gasohol.
Define the term average bond enthalpy.
Use the information from Table 10 of the Data Booklet to determine the standard enthalpy change for the complete combustion of ethanol.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH(g)}} + {\text{3}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\]
The standard enthalpy change for the complete combustion of octane, \({{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{18}}}}\), is \( - 5471{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the amount of energy produced in kJ when 1 g of ethanol and 1 g of octane is burned completely in air.
Ethanol can be oxidized using acidified potassium dichromate, \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\), to form two different organic products.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\xrightarrow[{{{\text{H}}^ + }}]{{{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 - }}}\) A \(\xrightarrow[{{{\text{H}}^ + }}]{{{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 - }}}\) B
State the structural formulas of the organic products A and B and describe the conditions required to obtain a high yield of each of them.
Deduce and explain whether ethanol or A has the higher boiling point.
Ethene can be converted into ethanol by direct hydration in the presence of a catalyst according to the following equation.
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{H}}_{\text{2}}}{\text{O(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH(g)}}\]
For this reaction identify the catalyst used and state one use of the ethanol formed other than as a fuel.
State the name of one structural isomer of pentane.
Markscheme
energy required to break (1 mol of) a bond in a gaseous molecule/state;
Accept energy released when (1 mol of) a bond is formed in a gaseous molecule/state / enthalpy change when (1 mol of) bonds are made or broken in the gaseous molecule/state.
average values obtained from a number of similar bonds/compounds / OWTTE;
Bonds broken
\({\text{(1)(C}}–{\text{C)}} + {\text{(1)(O}}–{\text{H)}} + {\text{(5)(C}}–{\text{H)}} + {\text{(1)(C}}–{\text{O)}} + {\text{(3)(O=O)}}\)
\( = (1)(347) + (1)(464) + (5)(413) + (1)(358) + (3)(498) = 4728{\text{ (kJ)}}\);
Bonds formed
\({\text{(2}} \times {\text{2)(C=O)}} + {\text{(3}} \times {\text{2)(O}}–{\text{H)}}\)
\( = (4)(746) + (6)(464) = 5768{\text{ (kJ)}}\);
\(\Delta H = 4728 - 5768 = - 1040{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}/ - 1040{\text{ kJ}}\);
Units needed for last mark.
Award [3] for final correct answer.
Award [2] for +1040 kJ.
\({M_{\text{r}}}{\text{(}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH)}} = 46.08/46.1\) and \({M_{\text{r}}}{\text{(}}{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{18}}}}{\text{)}} = 114.26/114.3\);
1ethanol produces 22.57 kJ and 1octane produces 47.88 kJ;
Accept values ranges of 22.5–23 and 47.8–48 kJ respectively.
No penalty for use of Mr = 46 and Mr = 114.
A: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\);
B: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Accept either full or condensed structural formulas but not the names or molecular formulas.
A: distillation;
B: reflux;
ethanol/; \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
hydrogen bonding (in ethanol);
Award second point only if the first is obtained.
(concentrated) \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\)/(concentrated) phosphoric acid / \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\)/sulfuric acid;
dyes / drugs / cosmetics / solvent / (used to make) esters / (used in) esterification/disinfectant;
(2-)methylbutane / (2,2-)dimethylpropane;
Examiners report
The definition of average bond enthalpy given by most candidates was not complete in (a) (i). The word gaseous was missing and the fact that it is an average of values from bonds in similar compounds was very rarely mentioned.
In (ii) the calculation of the standard enthalpy change for the combustion of ethanol was done correctly by most candidates.
In (a) (iii) the amount of energy produced by 1g of ethanol and by 1g of octane was correctly calculated by some of the candidates.
Candidates gave correct formulas for the aldehyde and the carboxylic acid in (iv), but the conditions required to obtain a high yield were not correctly stated or were absent.
In (a) (v) most candidates correctly stated that ethanol would have a higher boiling point than ethanal because of the presence of hydrogen bonding in ethanol.
In (vi) the catalyst for the conversion of ethane into ethanol was not always identified.
In (b)(i) most candidates stated correctly that methylbutane would be a structural isomer of pentane.
To determine the enthalpy change of combustion of methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\), 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water from 24.5 °C to 45.8 °C.
The manufacture of gaseous methanol from CO and \({{\text{H}}_{\text{2}}}\) involves an equilibrium reaction.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{OH(g)}}\,\,\,\,\,\Delta {H^\Theta } < 0\]
State and explain the effect of the following changes on the equilibrium position of the reaction in part (c).
Calculate the enthalpy change of combustion of methanol.
Using the theoretical value in Table 12 of the Data Booklet, discuss the experimental results, including one improvement that could be made.
Methanol can be produced according to the following equation.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{OH(l)}}\]
Calculate the standard enthalpy change of this reaction using the following data:
\[\begin{array}{*{20}{l}} {{\text{I: 2C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1452{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{II: 2CO(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta {H^\Theta } = - 566{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{III: 2}}{{\text{H}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Outline the characteristics of a chemical equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for this reaction.
Increase in temperature.
Increase in pressure.
Addition of a catalyst.
Markscheme
\((q = mc\Delta T = ){\text{ }}0.0500 \times 4.18 \times 21.3 = 4.45{\text{ (kJ)}}\);
Do not accept m = 0.05023 kg.
\({\text{(}}n{\text{ }}methanol = {\text{) }}\frac{{0.230}}{{{\text{32.05}}}} = 7.18 \times {10^{ - 3}}{\text{ (mol)}}\);
\(\Delta H = \frac{{4.45}}{{7.18 \times {{10}^{ - 3}}}}\);
\(\Delta H = - 6.20 \times {10^2}{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Accept integer values of molar mass.
Final answer must have negative sign and correct units.
Award [4] for correct final answer with correct units.
less heat is liberated than theoretically/\( - 726{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
probably due to heat loss/incomplete combustion;
determine heat capacity of calorimeter and take heat absorbed by calorimeter into account / any suitable insulation method / measure temperature with time and extrapolation of graph to compensate heat loss / OWTTE;
If the value calculated in (a) (i) is more exothermic than theoretically, allow ECF for M1 and for improvement if consistent.
\(\Delta {H^\Theta } = \frac{1}{2}{\text{II}} + {\text{III}} - \frac{1}{2}{\text{I}}\) / correct diagram/energy cycle;
\( - 283 - 572 - ( - 726)\);
\( - 129{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
rate of forward reaction equals rate of backward reaction;
concentrations of reactants and products do not change / constant macroscopic properties;
\({K_{\text{c}}} = \frac{{{\text{[C}}{{\text{H}}_3}{\text{OH]}}}}{{{\text{[CO][}}{{\text{H}}_2}{{\text{]}}^2}}}\);
Do not award mark if incorrect brackets are used or brackets omitted.
shifts to left/reactants;
to endothermic side / (forward) reaction is exothermic;
shifts to the right/products;
to the side with fewer gas molecules/moles of gas;
no effect on equilibrium;
rate of forward and backward reaction increase equally / activation energy of forward and backward reaction lowered equally;
Examiners report
Many candidates used the mass of methanol in their calculation and most did not convert the mass of methanol to moles.
Students did not make a comparison between their calculated value and the theoretical value, often just stating the numbers. Most candidates were aware that heat was lost but improvements were generally simplistic.
The energy cycle was fairly well done, though working out could be shown better.
Many students had no problem with the characteristics of a chemical equilibrium.
The expression for\({K_{\text{c}}}\) was done quite well.
The effect of changes on the equilibrium position was answered quite well, though candidates had difficulty in explaining the rationale, omitting often gas molecules (ii) and increasing equally in (iii).
The effect of changes on the equilibrium position was answered quite well, though candidates had difficulty in explaining the rationale, omitting often gas molecules (ii) and increasing equally in (iii).
The effect of changes on the equilibrium position was answered quite well, though candidates had difficulty in explaining the rationale, omitting often gas molecules (ii) and increasing equally in (iii).
Define the term activation energy, \({E_{\text{a}}}\).
State two conditions necessary for a reaction to take place between two reactant particles.
Sketch an enthalpy level diagram to describe the effect of a catalyst on an exothermic reaction.
Markscheme
(minimum) energy needed for a reaction to occur / (minimum) energy difference between reactants and transition state;
particles must collide;
appropriate collision geometry/orientation;
\(E \geqslant {E_{\text{a}}}\);
Diagram showing:
correct labelling of axes (enthalpy/H/(potential) energy for y-axis and time/progress/course of reaction/reaction coordinate for x-axis) and H (products) line shown below H (reactants) line;
correct labelling of the two curves, catalysed and uncatalysed;
correct position of \({E_{\text{a}}}\) shown with lines for a catalysed and uncatalysed reaction;
the correct label \(\Delta H\) /change in enthalpy;
Do not penalize if reactants and products are not labelled.
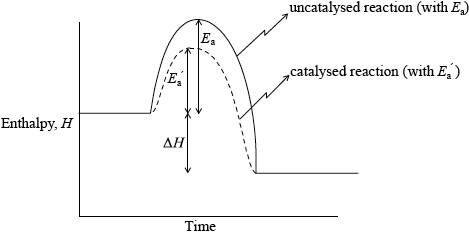
If an endothermic reaction is shown, award [2 max] if all other parts are shown correctly.
Examiners report
Most candidates gave the correct definition of activation energy in (a).
The two conditions needed for a reaction to take place were given by the majority of candidates.
In (c) some of the enthalpy level diagrams had many labels missing. Axes weren‟t always labelled, one of them was wrongly labelled as delta H, and the curves of Ea with and without catalyst were not properly indicated. A few answers showed an endothermic reaction instead.
Alkenes, such as A (shown below), are important intermediates in the petrochemical industry because they undergo addition reactions to produce a wide variety of products, such as the conversion shown below.
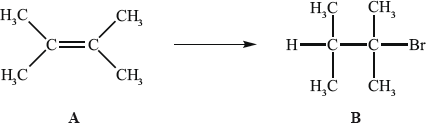
Another way to make B is the reaction shown below.
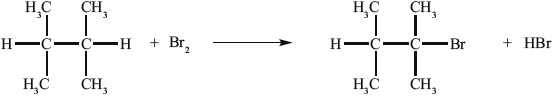
B can be converted into C.

In the gas phase, A reacts with hydrogen to form D.
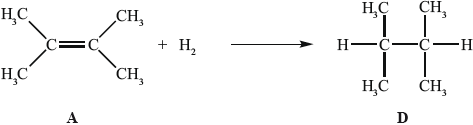
Applying IUPAC rules, state the name of A.
State the reagent required to convert A into B.
(i) State the conditions required for this reaction to occur.
(ii) Outline why it would give a poor yield of the desired product.
(i) State the reagent required.
(ii) Explain the mechanism of this reaction, using curly arrows to represent the movement of electron pairs.
A can also be converted into C without going via B. State the reagent and conditions required.
(i) State why C is not readily oxidized by acidified potassium dichromate(VI).
(ii) Deduce the structural formula of an isomer of C that could be oxidized to a carboxylic acid by this reagent.
State the conditions required for this reaction to occur.
State the homologous series to which D belongs.
Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of A with hydrogen, using Table 10 of the Data Booklet, and state whether the reaction is exothermic or endothermic.
The standard enthalpy change of combustion of A is \( - 4000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the amount of A, in mol, that would have to be burned to raise the temperature of \({\text{1 d}}{{\text{m}}^{\text{3}}}\) of water from 20 °C to 100 °C.
Markscheme
2,3-dimethylbut-2-ene;
Ignore punctuation.
hydrogen bromide / hydrobromic acid / HBr;
(i) ultraviolet light/sunlight;
Accept “very high temperature”.
(ii) random/further/multiple substitution (so low probability of desired product) / would give a mixture of many different products / OWTTE;
(i) (aqueous) sodium hydroxide/NaOH / potassium hydroxide/KOH;
Accept hydroxide ion/OH–.
(ii) 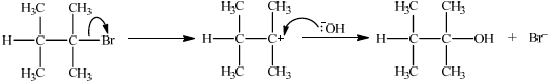
\({S_N}1\):
curly arrow from C–Br bond showing Br leaving;
representation of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in HO–.
Award [2] for perfect SN2 mechanism.
Award [1] for SN2 mechanism with minor mistakes.
water / steam;
heat and acid catalyst /(concentrated) \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}/{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\);
(i) (it is a) tertiary/3° alcohol / carbon of C–OH is not bonded to a hydrogen;
Accept “it is not a primary or secondary alcohol”.
(ii) any \({{\text{C}}_6}{{\text{H}}_{14}}{\text{O}}\) primary alcohol / \({{\text{C}}_5}{{\text{H}}_{11}}{\text{C}}{{\text{H}}_2}{\text{OH}}\);
Ni/Pt/Pd catalyst;
alkanes;
bonds broken: (E(C=C) + E(H–H) = 612 + 436 =) \({\text{1048 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept (6956 + 436 =) 7392 if all bonds in alkene broken.
bonds formed: E(C–C) + 2 \( \times \) E(C–H) = 347 + (2 \( \times \) 413) = \({\text{1173 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept 7517 if all the bonds in the product are summed.
\(\Delta H = 1048 - 1173/7392 - 7517 = - 125{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +125.
exothermic;
Apply ECF if sign of \(\Delta H\) incorrect.
Do not award a mark for “exothermic” if \(\Delta H\) given as positive.
energy required to heat water \(\left( { = m \times s \times \Delta T = 1 \times 4.18 \times (100 - 20)} \right) = 334.4{\text{ }}({\text{kJ}})\);
Ignore sign of energy change.
amount required \(\frac{{334.4}}{{4000}} = 0.0836{\text{ (mol)}}\);
Award [2] for correct final answer.
Examiners report
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Two groups of students (Group A and Group B) carried out a project* on the chemistry of some group 7 elements (the halogens) and their compounds.
* Adapted from J Derek Woollins, (2009), Inorganic Experiments and Open University, (2008), Exploring the Molecular World.
In the first part of the project, the two groups had a sample of iodine monochloride (a corrosive brown liquid) prepared for them by their teacher using the following reaction.
\[{{\text{I}}_{\text{2}}}{\text{(s)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{2ICl(l)}}\]
The following data were recorded.
The students reacted ICl(l) with CsBr(s) to form a yellow solid, \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), as one of the products. \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) has been found to produce very pure CsCl(s) which is used in cancer treatment.
To confirm the composition of the yellow solid, Group A determined the amount of iodine in 0.2015 g of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) by titrating it with \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\). The following data were recorded for the titration.

(i) State the number of significant figures for the masses of \({{\text{I}}_{\text{2}}}{\text{(s)}}\) and ICl(l).
\({{\text{I}}_{\text{2}}}{\text{(s)}}\):
ICl (l):
(ii) The iodine used in the reaction was in excess. Determine the theoretical yield, in g, of ICl(l).
(iii) Calculate the percentage yield of ICl(l).
(iv) Using a digital thermometer, the students discovered that the reaction was exothermic. State the sign of the enthalpy change of the reaction, \(\Delta H\).
Although the molar masses of ICl and \({\rm{B}}{{\rm{r}}_2}\) are very similar, the boiling point of ICl is 97.4 °C and that of \({\rm{B}}{{\rm{r}}_2}\) is 58.8 °C. Explain the difference in these boiling points in terms of the intermolecular forces present in each liquid.
(i) Calculate the percentage of iodine by mass in \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures.
(ii) State the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) used in the titration.
(iii) Determine the amount, in mol, of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) added in the titration.
(iv) The overall reaction taking place during the titration is:
\[{\text{CsICl(s)}} + {\text{2N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaCl(aq)}} + {\text{N}}{{\text{a}}_2}{{\text{S}}_4}{{\text{O}}_6}{\text{(aq)}} + {\text{CsCl(aq)}} + {\text{NaI(aq)}}\]
Calculate the amount, in mol, of iodine atoms, I, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\).
(v) Calculate the mass of iodine, in g, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}\)
(vi) Determine the percentage by mass of iodine in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures, using your answer from (v).
Markscheme
(i) I2(s): four/4 and ICl(l): three/3;
(ii) \(n{\text{(C}}{{\text{l}}_2}{\text{)}} = {\text{ }}\left( {\frac{{2.24}}{{2 \times 35.45}} = } \right){\text{ }}0.0316/3.16 \times {10^{ - 2}}{\text{ (mol)}}\);
Allow answers such as 3.2 \( \times \) 10–2/0.032/3.15 \( \times \) 10–2/0.0315 (mol).
\(n{\text{(ICl)}} = 2 \times 0.0316/0.0632/6.32 \times {10^{ - 2}}{\text{ (mol)}}\);
Allow answers such as 6.4 \( \times \) 10–2/0.064/6.3 \( \times \) 10–2/0.063 (mol).
\(m{\text{(ICl)}} = (0.0632 \times 162.35 = ){\text{ }}10.3{\text{ (g)}}\);
Allow answers in range 10.2 to 10.4 (g).
Award [3] for correct final answer.
(iii) \(\left( {\frac{{8.60}}{{10.3}} \times 100 = } \right){\text{ }}83.5\% \);
Allow answers in the range of 82.5 to 84.5%.
(iv) negative/–/minus/ < 0;
Br2 has London/dispersion/van der Waals’ forces/vdW and ICl has (London/dispersion/van der Waals’ forces/vdW and) dipole–dipole forces;
dipole–dipole forces are stronger than London/dispersion/van der Waals’/vdW forces;
Allow induced dipole-induced dipole forces for London forces.
Allow interactions instead of forces.
Do not allow ICl polar and Br2 non-polar for M1.
Name of IMF in both molecules is required for M1 and idea of dipole-dipole stronger than vdW is required for M2.
(i) \(\left( {\frac{{126.90}}{{330.71}} \times 100} \right) = 38.4\% \);
(ii) \((25.25 - 1.05) = 24.20{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept 24.2 (cm3) but not 24 (cm3).
(iii) \(\left( {\frac{{24.20 \times 5.00 \times {{10}^{ - 2}}}}{{1000}}} \right) = 1.21 \times {10^{ - 3}}/0.00121{\text{ (mol)}}\);
(iv) \((0.5 \times 1.21 \times {10^{ - 3}}) = 6.05 \times {10^{ - 4}}/0.000605{\text{ (mol)}}\);
Accept alternate method e.g. (0.384/126.9 \( \times \) 0.2015) = 6.10 \( \times \) 10–4/0.000610 (mol).
(v) \((126.90 \times 6.05 \times {10^{ - 4}}) = 7.68 \times {10^{ - 2}}/0.0768{\text{ (g)}}\);
Accept alternate method e.g. (6.10 \( \times \) 10–4 \( \times \) 126.9) or (0.2015 \( \times \) 0.384) = 7.74 \( \times \) 10–2/0.00774 (g).
(vi) \(\left( {\frac{{7.68 \times {{10}^{ - 2}}}}{{0.2015}} \times 100} \right) = 38.1\% \);
Answer must be given to three significant figures.
Examiners report
This was a data based question based on quantitative chemistry. Majority of candidates were able to gain almost full marks with some candidates failing to recognise that chlorine is the limiting reagent in part (a) (ii). Some candidates calculated percentage experimental error instead of percentage yield whereas some other candidates did not pay attention to significant digits.
In part (b), explaining the difference in the boiling points of Br2 and ICl in terms of the intermolecular forces presented a challenge to many candidates. Explanations were vague or unclear and in some cases incorrect in terms of the intermolecular forces present.
In part (c), calculations of moles of iodine occasionally saw the erroneous use of Avogadro’s constant.
Chlorine occurs in Group 7, the halogens.
Two stable isotopes of chlorine are \(^{{\text{35}}}{\text{Cl}}\) and \(^{{\text{37}}}{\text{Cl}}\) with mass numbers 35 and 37 respectively.
Chlorine has an electronegativity value of 3.2 on the Pauling scale.
Chloroethene, H2C=CHCl, the monomer used in the polymerization reaction in the manufacture of the polymer poly(chloroethene), PVC, can be synthesized in the following two-stage reaction pathway.
\[\begin{array}{*{20}{l}} {{\text{Stage 1:}}}&{{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}}} \\ {{\text{Stage 2:}}}&{{\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}} + {\text{HC=CHCl(g)}} + {\text{HCl(g)}}} \end{array}\]
Define the term isotopes of an element.
Calculate the number of protons, neutrons and electrons in the isotopes 35Cl and 37Cl.
Using the mass numbers of the two isotopes and the relative atomic mass of chlorine from Table 5 of the Data Booklet, determine the percentage abundance of each isotope.
Percentage abundance 35Cl:
Percentage abundance 37Cl:
Define the term electronegativity.
Using Table 7 of the Data Booklet, explain the trends in electronegativity values of the Group 7 elements from F to I.
State the balanced chemical equation for the reaction of potassium bromide, KBr(aq), with chlorine, Cl2(aq).
Describe the colour change likely to be observed in this reaction.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for stage 1 using average bond enthalpy data from Table 10 of the Data Booklet.
State whether the reaction given in stage 1 is exothermic or endothermic.
Draw the structure of poly(chloroethene) showing two repeating units.
Suggest why monomers are often gases or volatile liquids whereas polymers are solids.
Markscheme
atoms of same element / atoms with same number of protons/atomic number/Z;
Do not allow elements instead of atoms in second alternative.
(but) different numbers of neutrons/mass number/A;
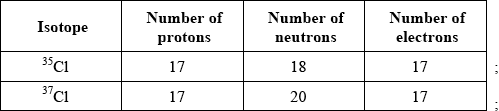
Allow [1 max] for 17 p, 17 e for both if n’s are omitted or incorrect.
Allow [1 max] for 35Cl: 18 n and 37Cl: 20 n if p’s and e’s are omitted.
\(({\text{for}}{{\text{ }}^{{\text{35}}}}{\text{Cl}}:x\% ){\text{ }}35x + 3700 - 37x = 3545\);
Allow other alternative mathematical arrangements.
\(^{{\text{35}}}{\text{Cl}} = 77.5\% \) and \(^{{\text{37}}}{\text{Cl}} = 22.5\% \);
Award [1 max] for correct percentages if no correct working is shown.
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons / OWTTE;
Do not allow element instead of atom/nucleus.
increasing atomic radii (down the group) / OWTTE;
so reduced attraction (for the bonding electrons) / OWTTE;
screening/shielding effect of inner electrons / OWTTE;
Allow more energy levels/electron shells for M1.
Do not accept decrease in nuclear charge.
\({\text{2KBr(aq)}} + {\text{C}}{{\text{l}}_2}{\text{(aq)}} \to {\text{2KCl(aq)}} + {\text{B}}{{\text{r}}_2}{\text{(aq)}}\);
Ignore state symbols.
Allow ionic equation.
colourless/pale yellow/green to yellow/orange/brown;
Start and end colours must both be mentioned.
Bonds breaking:
1 \( \times \) (C=C) \( + \) 4 \( \times \) (C–H) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (4)(413) + (1)(243)/ = ( + )2507{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 4 \( \times \) (C–H) \( + \) 2 \( \times \) (Cl–Cl)
\( = (1)(347) + (4)(413) + (2)(346)/ = - 2691{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((2507 - 2691 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
OR
Bonds breaking:
1 \( \times \) (C=C) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (1)(243)/ = ( + )855{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 2 \( \times \) (C–Cl)
\( = (1)(347) + (2)(346)/ = - 1039{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((855 - 1039 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
exothermic;
Do not award mark unless based on some value for part (iii).
representation of PVC showing two repeating units;
For example,
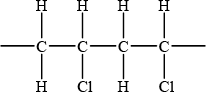
Brackets not necessary but continuation bonds must be given.
No penalty if chlorines are not on same side.
No penalty if chlorines are on two middle C atoms or on two end C atoms.
monomers are smaller molecules / monomers have smaller mass / smaller surface area than polymers;
weaker/fewer intermolecular/London/dispersion/van der Waals’ forces (of attraction);
Allow reverse argument.
Allow abbreviation for London/dispersion as FDL or for van der Waals’ as vdW.
Award zero if reference is made to breaking of bonds.
Examiners report
This was by far the most popular choice of question in Section B. Again, part a) (i) proved challenging as many candidates failed to refer to atoms in their definition and scored only 1 mark out of 2.
In a) (ii) most candidates could state the numbers of protons, neutrons and electrons in the isotopes of chlorine. Those who got this wrong gave answers which indicated a complete lack of understanding of atomic structure.
In a) (iii) some candidates remembered the percentage abundance of chlorine isotopes but could not do the calculation.
Part b) (i) required another definition. Again, many candidates lost marks for inarticulate responses.
The explanation in b) (ii) of trends in electronegativity values was reasonably well done, with most candidates scoring at least one mark out of two.
However, writing a balanced equation in b) (iii) was poorly done with many candidates not knowing the formula of KCl, and not knowing what products would be formed. This is clearly on the syllabus in 3.3.1.
Almost no-one knew the colours of aqueous chlorine and aqueous bromine in b) (iv).
In part c) (ii) the calculation of \(\Delta H\) using bond enthalpies was done well. Some candidates failed to use the C=C bond enthalpy value and some did not recall that bond breaking is endothermic and bond formation exothermic.
Nearly everyone scored a mark in c) (iii) as follow-through marks were awarded.
Drawing two repeating units of poly(chloroethene) presented difficulties in c) (iv). Some candidates tried to draw the monomers joined through the chlorine atoms.
In c) (v) most candidates scored at least one out of two for explaining why monomers have a much lower melting point than polymers.
Consider the following list of organic compounds.
Compound 1: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
Compound 2: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
Compound 3: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound 4: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = -57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Apply IUPAC rules to state the name of compound 1.
(i) Define the term structural isomers.
(ii) Identify the two compounds in the list that are structural isomers of each other.
Determine the organic product formed when each of the compounds is heated under reflux with excess acidified potassium dichromate(VI). If no reaction occurs write NO REACTION in the table.
Explain the mechanism for the substitution reaction of bromoethane with sodium hydroxide. Use curly arrows to represent the movement of electron pairs.
(i) Define the term standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Markscheme
butan-2-ol/2-butanol;
(i) same molecular formula but differ in arrangement of their atoms;
Allow “different structures/structural formulas” instead of “different arrangement of atoms”.
(ii) (compounds) 2 and 4 / butanone and butanal;
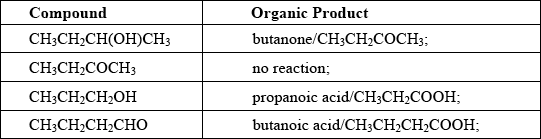
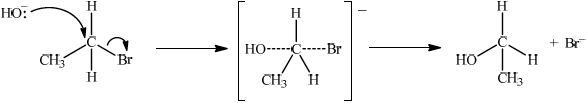
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented, but penalise wrong bonding once only.
formation of organic product \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Accept “NaBr / Na+ and Br–” as product.
If candidate writes an SN1 mechanism then deduct 1 mark for this, so that it is marked out of [3 max].
(i) heat transferred/absorbed/released/enthalpy/potential energy change when 1 mol/molar amounts of reactant(s) react (to form products) / OWTTE;
under standard conditions / at a pressure 100 kPa/101.3 kPa/1 atm and temperature 298 K/25 °C;
Award [2] for difference between standard enthalpies of products and standard enthalpies of reactants / \({H^\Theta }\) (products) – \({H^\Theta }\) (reactants).
Award [2] for difference between standard enthalpies of formation of products and standard enthalpies of formation of reactants / \(\Sigma \Delta H_f^\Theta \) (products) – \(\Sigma \Delta H_f^\Theta \) (reactants).
(ii) \((1.00 \times 0.0500 = ){\text{ }}0.0500{\text{ (mol)}}\);
\((0.0500 \times 57.9 = ){\text{ }}2.90{\text{ (kJ)}}\);
Ignore any negative sign.
Award [2] for correct final answer.
Award [1 max] for 2900 J.
(iii) \(\left( {\frac{{2.50}}{{40.00}} = } \right){\text{ }}0.0625{\text{ (mol NaOH)}}\);
\(0.0500 \times 4.18 \times 13.3 = 2.78{\text{ (kJ)}}/50.0 \times 4.18 \times 13.3 = 2780{\text{ (J)}}\);
\(\left( {\frac{{2.78}}{{0.0625}}} \right) = - 44.5{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Negative sign is necessary for M3.
Award M2 and M3 if 52.5 g is used to obtain an enthalpy change of –46.7.
(iv) –44.5 – 57.9 / correct Hess’s Law cycle (as below) / correct manipulation of equations;
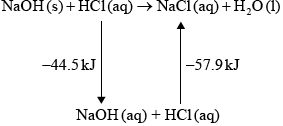
–102.4 (kJ);
Award [2] for correct final answer.
Examiners report
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
0.100 g of magnesium ribbon is added to \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid to produce hydrogen gas and magnesium sulfate.
\[{\text{Mg(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {{\text{H}}_2}{\text{(g)}} + {\text{MgS}}{{\text{O}}_4}{\text{(aq)}}\]
Magnesium sulfate can exist in either the hydrated form or in the anhydrous form. Two students wished to determine the enthalpy of hydration of anhydrous magnesium sulfate. They measured the initial and the highest temperature reached when anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}{\text{(s)}}\), was dissolved in water. They presented their results in the following table.

The students repeated the experiment using 6.16 g of solid hydrated magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}} \bullet {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(s)}}\), and \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. They found the enthalpy change, \(\Delta {H_2}\), to be \( + 18{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The enthalpy of hydration of solid anhydrous magnesium sulfate is difficult to determine experimentally, but can be determined using the diagram below.
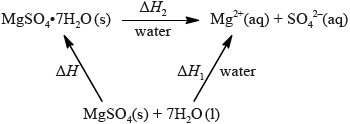
Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic limestone. This limestone is a mixture of magnesium carbonate and calcium carbonate.
(i) The graph shows the volume of hydrogen produced against time under these experimental conditions.
Sketch two curves, labelled I and II, to show how the volume of hydrogen produced (under the same temperature and pressure) changes with time when:
I. using the same mass of magnesium powder instead of a piece of magnesium ribbon;
II. 0.100 g of magnesium ribbon is added to \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid.
(ii) Outline why it is better to measure the volume of hydrogen produced against time rather than the loss of mass of reactants against time.
(i) Calculate the amount, in mol, of anhydrous magnesium sulfate.
(ii) Calculate the enthalpy change, \(\Delta {H_1}\), for anhydrous magnesium sulfate dissolving in water, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). State your answer to the correct number of significant figures.
(i) Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the hydration of solid anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}\).
(ii) The literature value for the enthalpy of hydration of anhydrous magnesium sulfate is \( - 103{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage difference between the literature value and the value determined from experimental results, giving your answer to one decimal place. (If you did not obtain an answer for the experimental value in (c)(i) then use the value of \( - 100{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Another group of students experimentally determined an enthalpy of hydration of \( - 95{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Outline two reasons which may explain the variation between the experimental and literature values.
(i) State the equation for the reaction of sulfuric acid with magnesium carbonate.
(ii) Deduce the Lewis (electron dot) structure of the carbonate ion, giving the shape and the oxygen-carbon-oxygen bond angle.
Lewis (electron dot) structure:
Shape:
Bond angle:
Markscheme
(i) 
I: line which is steeper/increases faster and finishes at the same height;
II: line which is less steep/increases more slowly and finishes at the same height;
(ii) mass of hydrogen produced is very small (so not accurate) / decrease in mass is very small (so not accurate);
(i) \(n({\text{MgS}}{{\text{O}}_4}) = \left( {\frac{{3.01}}{{120.37}} = } \right){\text{ }}0.0250{\text{ (mol)}}\);
(ii) energy released \( = 50.0 \times 4.18 \times 9.7 \times 2027{\text{ (J)}}/2.027{\text{ (kJ)}}\);
\(\Delta {H_1} = - 81{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [2] for correct answer.
Award [2] if 53.01 is used giving an answer of –86 (kJ mol–1).
Award [1 max] for +81/81/+86/86 (kJ mol−1).
Award [1 max] for –81000/–86000 if units are stated as J mol−1.
Allow answers to 3 significant figures.
(i) \(\Delta H{\text{ }}( = \Delta {H_1} - \Delta {H_2}) = - 99{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [1] if –86 is used giving an answer of –104 (kJ mol−1).
(ii) \(\frac{{(103 - 99)}}{{103}} \times 100 = 3.9\% \);
Accept answer of 2.9 % if –100 used but only if a value for (b)(i) is not
present.
Award [1] if –104 is used giving an answer of 1.0% .
Accept correct answers which are not to 1 decimal place.
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) not completely anhydrous / OWTTE;
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) is impure;
heat loss to the atmosphere/surroundings;
specific heat capacity of solution is taken as that of pure water;
experiment was done once only so it is not scientific;
density of solution is taken to be \(1{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\);
mass of \(7{{\text{H}}_2}{\text{O}}\) ignored in calculation;
uncertainty of thermometer is high so temperature change is unreliable;
literature values determined under standard conditions but this experiment is not;
all solid not dissolved;
(i) \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{MgC}}{{\text{O}}_3}{\text{(s)}} \to {\text{MgS}}{{\text{O}}_4}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
Do not accept H2CO3.
(ii) 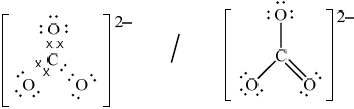 ;
;
Accept crosses, lines or dots as electron pairs.
Accept any correct resonance structure.
Award [0] if structure is drawn without brackets and charge.
Award [0] if lone pairs not shown on O atoms.
shape: trigonal/triangular planar;
bond angle: 120°;
Accept answers trigonal/triangular planar and 120° if M1 incorrect, but no other answer should be given credit.
Examiners report
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Ethanol has many industrial uses.
(i) State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
(ii) Deduce the volume of ethanol, in dm3, produced from \({\text{1.5 d}}{{\text{m}}^{\text{3}}}\) of ethene, assuming both are gaseous and at the same temperature and pressure.
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Suggest why the value of the enthalpy of combustion of ethanol quoted in table 12 of the data booklet is different to that calculated using bond enthalpies.
Explain why the reaction is exothermic in terms of the bonds involved.
Identify the homologous series to which ethanol belongs and state two features of a homologous series.
Markscheme
(i) Equation:
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}/{{\text{C}}_2}{{\text{H}}_4} + {{\text{H}}_2}{\text{O}} \to {{\text{C}}_2}{{\text{H}}_5}{\text{OH}}\);
Conditions:
(concentrated) sulfuric acid/\({{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{4}}}\);
Do not accept dilute sulfuric acid.
Accept phosphoric acid/H3PO4 (on pellets of silicon dioxide) (for industrial preparation).
heat / high temperature;
Do not accept warm.
Accept high pressure (for industrial preparation) for M3 only if H3PO4 is given for M2.
(ii) \({\text{1.5 (d}}{{\text{m}}^{\text{3}}}{\text{)}}\);
energy needed to break (1 mol of) a bond in the gaseous state/phase;
(averaged over) similar compounds;
Do not accept “similar bonds” instead of “similar compounds”.
Concept of “similar” is important for M2.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{3}}{{\text{O}}_2} \to {\text{2C}}{{\text{O}}_2} + {\text{3}}{{\text{H}}_2}{\text{O}}\);
Bonds broken:
\({\text{347}} + {\text{(5}} \times {\text{413)}} + {\text{358}} + {\text{464}} + {\text{(3}} \times {\text{498)}}/{\text{4728 (kJ)}}/\)
C–C + 5C–H + C–O + O–H + 3O=O;
Bonds made:
\((4 \times 746) + (6 \times 464)/5768{\text{ (kJ)}}/\) 4C=O + 6O—H;
\(\Delta H = (4728 - 5768 = ) - 1040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\) / bonds broken − bonds formed;
Award [4] for correct final answer.
Award [3] for (+)1040 (kJ mol–1).
ethanol and water are liquids / not all molecules are gaseous / in enthalpy of combustion molecules are in their standard states / bond enthalpies are average values;
Do not accept answer “ethanol/water is a liquid” alone.
less energy required to break bonds in reactants than is released when the bonds in products form / bonds stronger (overall) in products/weaker (overall) in reactants;
alcohols / alkanols;
Any two of the following for [2 max]:
differ by CH2/methylene (unit);
similar chemical properties;
gradually changing physical properties;
same general formula;
same functional group;
Do not accept “same” instead of “similar”, or vice-versa.
Examiners report
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success, although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success, although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success, although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success, although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
This was not a popular question with few candidates choosing it. Some who chose it did very well but most scored poorly. Students needed to write an equation for the hydration of ethene which was generally answered well and then state the conditions, which were less well known. Applying Avogadro's law to work out the volume of ethanol was only correctly answered by a few. The definition for bond enthalpy was not well known, however many candidates could calculate the energy change using bond enthalpies with some success, although there were few completely correct answers as bonds were forgotten or incorrectly multiplied.
Ethene, C2H4, and hydrazine, N2H4, are hydrides of adjacent elements in the periodic table.
The polarity of a molecule can be explained in terms of electronegativity.
The reaction between N2H4(aq) and HCl (aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw Lewis (electron dot) structures for \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) and \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) showing all valence electrons.
(ii) State and explain the H–C–H bond angle in ethene and the H–N–H bond angle in hydrazine.
(i) Define the term electronegativity.
(ii) Compare the relative polarities of the C–H bond in ethene and the N–H bond in hydrazine.
(iii) Hydrazine is a polar molecule and ethene is non-polar. Explain why ethene is non-polar.
The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
Hydrazine is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(g)}}\]
Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
State the name of the product and identify the type of reaction which occurs between ethene and hydrogen chloride.
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_6^{2 + }\).
Markscheme
(i) 
Accept x’s, dots or lines for electron pairs
(ii) H–C–H:
any angle between 118° and 122°;
due to three negative charge centres/electron domains/electron pairs;
H–N–H:
any angle between 104° and 108°;
due to four negative charge centres/electron domains/electron pairs;
extra repulsion due to lone electron pairs;
Do not allow ECF for wrong Lewis structures.
(i) (relative) measure of an atoms attraction for electrons;
in a covalent bond / shared pair;
(ii) C–H is less polar as C is less electronegative / N–H bond is more polar as N is more electronegative / difference in electronegativity is greater for N-H than C-H;
(iii) bond polarities cancel in \({{\text{C}}_2}{{\text{H}}_4}\) / OWTTE;
weaker van der Waals’/London/dispersion/intermolecular forces in ethene;
stronger (intermolecular) hydrogen bonding in hydrazine;
If no comparison between strengths then [1 max].
bonds broken: 4 N–H, N–N, O=O / \( + {\text{2220 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
bonds formed: N\(\equiv\)N, 4O–H / \( - 2801{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\( - 581{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
chloroethane;
(electrophilic) addition;
Do not accept free radical/nucleophilic addition.
(i) acid-base/neutralization;
(ii) 109°/109.5°;
Examiners report
This was a popular question and was answered quite successfully. The Lewis structure for ethene was given correctly by the great majority of the candidates, but that of hydrazine by only about half of them. Incorrect answers had double bonds appearing between the 2 nitrogen atoms and lone pairs on nitrogen atoms not shown. Those who could draw the correct structure in (i) gave the correct bond angle, but the explanation was not given correctly by many. Only very few scored the five marks as many failed to mention the extra repulsion of the lone pair.
The definition of electronegativity was not well known and many forgot to mention covalent bond or got confused with ionization and electron affinity and talked about a mole of gaseous atoms.
In part (c) most knew that hydrogen bonding in hydrazine was stronger than the van der Waals’ forces in ethene and explained its higher boiling point. However, some candidates described hydrogen bonding as the bond between N and H in the molecule, and some omitted a comparison of the relative strengths.
The calculation for the enthalpy change produced some completely correct calculations but many candidates lost marks here for using the wrong bond energies, although ECF was applied to the structures drawn in part (a).
In (e) ‘addition’ was correctly identified as the reaction type by most but when asked in (f) to identify the final reaction type few recognised it as an acid-base reaction, however, the bond angle was given correctly by many.
In (e) ‘addition’ was correctly identified as the reaction type by most but when asked in (f) to identify the final reaction type few recognised it as an acid-base reaction, however, the bond angle was given correctly by many.
In an experiment to measure the enthalpy change of combustion of ethanol, a student heated a copper calorimeter containing 100 cm3 of water with a spirit lamp and collected the following data.
\[\begin{array}{*{20}{l}} {{\text{Initial temperature of water:}}}&{{\text{20.0 }}^\circ {\text{C}}} \\ {{\text{Final temperature of water:}}}&{{\text{55.0 }}^\circ {\text{C}}} \\ {{\text{Mass of ethanol burned:}}}&{{\text{1.78 g}}} \\ {{\text{Density of water:}}}&{{\text{1.00 g}}\,{\text{c}}{{\text{m}}^{ - 3}}} \end{array}\]
(i) Use the data to calculate the heat evolved when the ethanol was combusted.
(ii) Calculate the enthalpy change of combustion per mole of ethanol.
(iii) Suggest two reasons why the result is not the same as the value in the Data Booklet.
Ethanol is part of the homologous series of alcohols. Describe two features of a homologous series.
(i) Below are four structural isomers of alcohols with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). State the name of each of the isomers a, b, c and D.
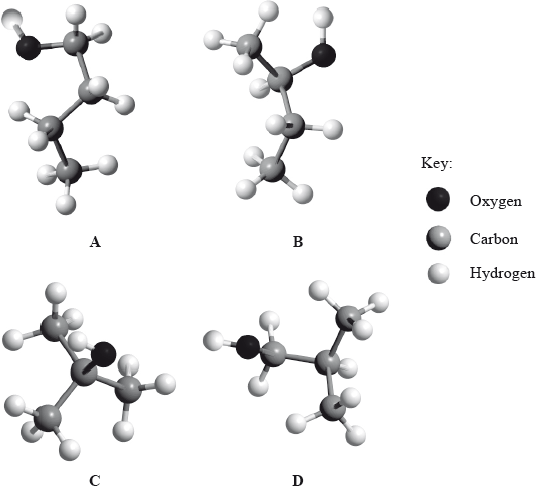
(ii) Determine the isomer that cannot be oxidized by acidifi ed potassium dichromate(VI), \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\).
(iii) Determine the isomer which can be oxidized to butanal.
(iv) Determine the isomer which can be oxidized to butanone.
(v) Suggest the structural formula of another isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\).

(i) Isomer a is formed by reacting 1-bromobutane with aqueous sodium hydroxide. State whether the reaction would proceed via an SN1 or SN2 mechanism.
(ii) Explain the mechanism named in part (d) (i) using curly arrows to represent the movement of electron pairs.
Markscheme
(i) \(100 \times 4.18 \times 35.0\);
14630 J / 14600 J / 14.6 kJ;
Award [2] for correct final answer.
No ECF here if incorrect mass used.
(ii) \(\frac{{1.78}}{{46.08}} = 0.0386{\text{ mol}}\);
\(\frac{{14.6}}{{0.0386}} = ( - )378{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Accept (–)377 and (–)379 kJ\(\,\)mol–1.
Award [2] for correct final answer.
(iii) heat loss;
incomplete combustion;
heat absorbed by calorimeter not included;
Accept other sensible suggestions.
same general formula;
same functional group;
successive members differ by CH2;
Allow methylene for CH2.
similar chemical properties;
gradually changing physical properties;
(i) A: butan-1-ol;
B: butan-2-ol;
C: (2-)methylpropan-2-ol;
D: (2-)methylpropan-1-ol;
Accept answers in the form of 1-butanol and 2-methyl-2-propanol etc.
Penalize incorrect punctuation, e.g. commas for hyphens, only once.
(ii) C/(2-)methylpropan-2-ol;
(iii) A/butan-1-ol;
(iv) B/butan-2-ol;
(v) 
(i) SN2;
(ii) 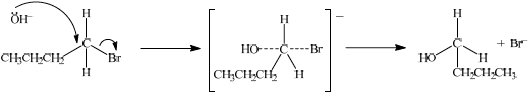
curly arrow going from lone pair/negative charge on O in \({\text{O}}{{\text{H}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 1-bromobutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
Examiners report
This was the least popular of the Section B questions. (a) (i) was poorly answered. Many candidates had no idea and some candidates used the mass of ethanol instead of water. A few calculated correctly but failed to convert the mass of water to kg, or kJ to J, thereby ending up with the wrong unit for the answer. Only a small minority of candidates got (ii) correct. (iii) was well answered. Nearly all candidates referred to heat loss but only the better candidates were able to give a second reason.
Most candidates were able to describe two features of a homologous series in (b).
(c) was usually well done, but some candidates struggled with the structural formula of the ether isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\) in (v).
One G2 comment stated that the ether functional group is not listed as one of the formal functional groups in Topic 10, which is correct. However, this aspect has been asked previously on SL papers in relation to deducing specific isomers (rather than naming the ether group) and although candidates are not required to know that C-O-C is the ether functional group, there is an expectation that they should be able to deduce an isomer based on C-O-C, as this is cited explicitly in AS 4.3.2, in the teacher’s notes in relation to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\), making this very much an objective 3 question, linking concepts across the syllabus.
SN2 was commonly given but the mechanism in (ii) was exceptionally poorly answered in this session. In particular, the transition state was rarely drawn, and clearly candidates were not prepared for organic reaction mechanisms, even though there are only a few such examples on the syllabus as a whole.
Some reactions of but-2-ene are given below.
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
State the names of the reagents D and E.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed directly from compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\).
(i) State the reagent and the conditions required for this reaction.
(ii) State the name of the type of reaction occurring in this conversion.
Compound C can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
Define the term average bond enthalpy.
Deduce the equation for the complete combustion of compound C.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the complete combustion of compound C when all reactants and products are in the gaseous state, using table 10 of the data booklet.
Markscheme
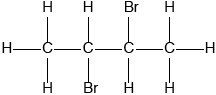 ;
;
Accept bromine atoms cis to each other.
2,3-dibromobutane;
Do not penalize the incorrect use of spaces, comma or hyphen.
red/brown/orange/yellow to colourless/decolourized;
Do not accept clear.
Do not accept just “decolourized”.
water;
sulfuric acid / phosphoric acid;
Accept formulas instead of names.
(i) (synthesis of) plastics/polymers/organic materials not naturally available / synthetic materials;
wide range of uses/physical properties / versatile;
large industry / many tons of plastics consumed by society / OWTTE;
Do not accept “useful” for M2.
Award [1 max] if specific addition polymer and its use is given.
Penalize reference to condensation polymers once only.
(ii) 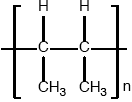 ;
;
Ignore n.
Brackets are not required for the mark, but continuation bonds are.
Do not penalize if methyl groups are trans to each other.
(i) aqueous sodium hydroxide/NaOH/potassium hydroxide/KOH and warm/heat/reflux;
(ii) (nucleophilic) substitution;
Accept (nucleophilic) displacement.
(i) carbonyl;
Accept ketone.
(ii)  ;
;
Accept condensed or full structural formula.
hydrogen bonding in compound C;
dipole-dipole forces in C / C is more polar;
C has greater molar mass/more dispersion/London/instantaneous induced dipole-induced dipole forces/van der Waal forces;
Accept converse argument.
Award [1 max] for stronger intermolecular forces.
energy required to break (1 mol of) a (covalent) bond in a gaseous molecule/state;
Accept energy released when (1 mol of) a (covalent) bond is formed in a gaseous molecule/state / energy change when (1 mol of) bonds are formed or broken in the gaseous molecule/state.
average value in similar compounds / OWTTE;
\({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{5}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\);
Ignore state symbols.
Bonds broken:
3C–C + 9C–H + 1C–O + 1O–H + 6O=O /
\(3 \times 347 + 9 \times 413 + 1 \times 358 + 1 \times 464 + 6 \times 498/8568{\text{ (kJ)}}\);
Bonds formed:
8C=O + 10O–H / \(8 \times 746 + 10 \times 464/10608{\text{ (kJ)}}\);
\(\Delta H = (8568 - 10608) = - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +2040 (kJ mol–1).
Examiners report
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
A student carried out an experiment to determine the concentration of a hydrochloric acid solution and the enthalpy change of the reaction between aqueous sodium hydroxide and this acid by thermometric titration.
She added \({\text{5.0 c}}{{\text{m}}^{\text{3}}}\) portions of hydrochloric acid to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution in a glass beaker until the total volume of acid added was \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\), measuring the temperature of the mixture each time. Her results are plotted in the graph below.
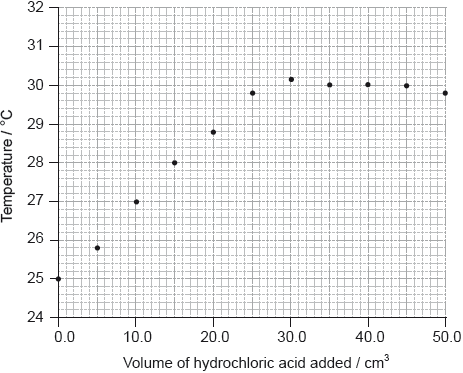
The initial temperature of both solutions was the same.
By drawing appropriate lines, determine the volume of hydrochloric acid required to completely neutralize the \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of sodium hydroxide solution.
Determine the concentration of the hydrochloric acid, including units.
Determine the change in temperature, \(\Delta T\).
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of hydrochloric acid and sodium hydroxide solution.
The accepted theoretical value from the literature of this enthalpy change is \( - 58{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage error correct to two significant figures.
Suggest the major source of error in the experimental procedure and an improvement that could be made to reduce it.
Markscheme
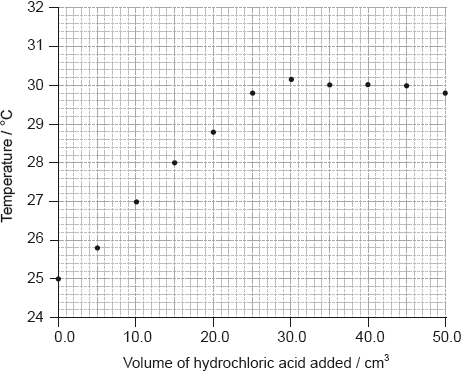
drawing best-fit straight lines to show volume;
There should be approximately the same number of points above and below for both lines.
\({\text{27.0 (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept any value in the range 26.0 to 28.0 (cm3) if consistent with student’s annotation on the graph.
Accept ECF for volumes in the range 27.0–30.0 cm3 if it corresponds to maximum temperature of line drawn.
Volumes should be given to one decimal place.
\({\text{[HCl]}} = \frac{{1.00 \times 0.0250}}{{0.0270}}\);
\( = 0.926{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
Volume of 26.0 gives [HCl] = 0.962 mol dm–3. Volume of 28.0 gives [HCl] = 0.893 mol dm–3
Award [2] for correct final answer with units.
Award [1 max] for correct concentration without units.
Accept M, mol L–1, mol/dm3 as units.
\((30.2 - 25.0 = )( + )5.2{\rm{ (^\circ C/K)}}\);
Any accepted value must be consistent with student’s annotation on the graph but do not accept \(\Delta T < 5.1\).
Accept \(( + )5.6{\rm{ (^\circ C/K) }}\) (ie, taking into account heat loss and using T when volume = 0.0 cm3).
\({\text{Q}} = (m \times c \times \Delta T = (25.0 + 27.0) \times 4.18 \times 5.2 = 1130.272{\text{ J}} = )1.13{\text{ (kJ)}}\);
\(n = (1.00 \times 0.0250 = )0.0250{\text{ (mol)}}\);
\(\Delta H = \left( { - \frac{Q}{n} = - 45210.88{\text{ J}}\,{\text{mo}}{{\text{l}}^{ - 1}} = } \right) - 45{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +45 (kJ mol –1).
Apply ECF for M3 even if both m and \(\Delta T\) are incorrect in M1.
Accept use of c = 4.2 Jg–1K–1.
\(\left( {\left| {\frac{{ - 45 - ( - 58)}}{{( - 58)}}} \right| \times 100 = } \right)22(\% )\);
Answer must be given to two significant figures.
Ignore sign.
heat losses;
better (thermal) insulation / using a polystyrene cup / putting a lid on the beaker;
Accept other suitable methods for better thermal insulation, but do not accept just "use a calorimeter" without reference to insulation.
Examiners report
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
The standard enthalpy change of three combustion reactions is given below in kJ.
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{7}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 3120} \\ {{\text{2}}{{\text{H}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 572} \\ {{{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1411} \end{array}\]
Based on the above information, calculate the standard change in enthalpy, \({\Delta {H^\Theta }}\), for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Markscheme
\(\begin{array}{*{20}{l}} {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}} \right)}&{\Delta {H^\Theta } = - 1560;} \\ {\left( {{{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + 286;} \\ {\left( {{\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + 1411;} \\ {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + 137{\text{ (kJ)}};} \end{array}\)
Allow other correct methods.
Award [2] for –137.
Allow ECF for the final marking point.
Examiners report
A significant number of candidates scored full marks here. Although the setting out of the calculations was often difficult to follow, a pleasing number of correct final answers was seen. The commonest errors were to give a correct numerical value with a negative sign and to fail to divide the value for the first equation by 2.
The standard enthalpy change of three combustion reactions are given below.
\[\begin{array}{*{20}{l}} {{{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 2219{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{C(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta H = - 394{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Determine the change in enthalpy, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the formation of propane in the following reaction.
\({\text{3C(s)}} + {\text{4}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_3}{{\text{H}}_8}{\text{(g)}}\)
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
Sketch two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_{\text{1}}}\) and \({T_2}{\text{ }}({T_2} > {T_1})\) and label both axes.

Markscheme
\({\text{4}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {\text{3C}}{{\text{O}}_2}{\text{(g)}} \to {{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}}\) \(\Delta H = + {\text{2219 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{4}}{{\text{H}}_2}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{4}}{{\text{H}}_2}{\text{O(l)}}\): \(\Delta H = \left( {{\text{(}} - {\text{286)(4)}} = } \right){\text{ }} - {\text{1144 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{3C(s)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}}\): \(\Delta H = \left( {{\text{(}} - {\text{394)(3)}} = } \right){\text{ }} - {\text{1182 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\Delta H = \left( {{\text{(}} - {\text{286)(4)}} + {\text{(}} - {\text{394)(3)}} + {\text{(}} + {\text{2219)}} = } \right){\text{ }} - {\text{107 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [4] for correct final answer.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction / for a successful collision;
Allow energy difference between reactants and transition state.
x-axis label: (kinetic) energy/(K)E and y-axis label: fraction of molecules/particles / probability density;
Allow velocity/speed for x-axis.
Allow frequency / number of molecules/particles or (kinetic) energy distribution for y-axis.
correct shape of a Maxwell–Boltzmann energy distribution curve;
Do not award mark if curve is symmetric, does not start at zero or if it crosses x-axis.
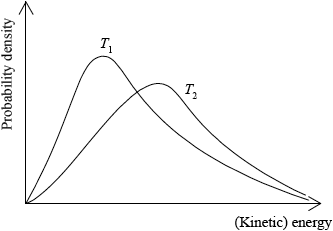
two curves represented with second curve for \({T_2} > {T_1}\) to right of first curve, lower
peak than first curve and after the curves cross \({T_2}\) curve needs to be above \({T_1}\) curve;
Examiners report
In contrast, question 2 a) which involved Hess’s Law calculation, was answered correctly by candidates of all capabilities.
The definition of activation energy in part b) was reasonably well answered, with some candidates losing marks for omitting the word minimum from their response. However, it is disappointing that even very good candidates sometimes fail to score marks for definitions.
Several candidates sketched very clear, correct Maxwell-Boltzmann curves in part c). Most scored at least 1 mark for this question. Some did not know what labels to put on the axes. Some did not realise that the area under the curves represents the total number of particles so as temperature increases the peak of the curve shifts to the right and is lower than the peak at the lower temperature.
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 g\(\,\)dm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) \( \rightleftharpoons \) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJ\(\,\)mol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJ\(\,\)mol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Markscheme
107°
Accept 100° to < 109.5°.
Literature value = 105.8°
[1 mark]
removes/reacts with OH−
moves to the right/products «to replace OH− ions»
Accept ionic equation for M1.
[2 marks]
N2H4(aq) + H2O(l) \( \rightleftharpoons \) N2H5+(aq) + OH–(aq)
Accept N2H4(aq) + 2H2O(l) \( \rightleftharpoons \) N2H62+(aq) + 2OH–(aq).
Equilibrium sign must be present.
[1 mark]
bubbles
OR
gas
OR
magnesium disappears
2NH4+(aq) + Mg(s) → Mg2+(aq) + 2NH3(aq) + H2(g)
Do not accept “hydrogen” without reference to observed changes.
Accept "smell of ammonia".
Accept 2H+(aq) + Mg(s) → Mg2+(aq) + H2(g)
Equation must be ionic.
[2 mark]
no oxygen required
[1 mark]
bonds broken:
E(N–N) + 4E(N–H)
OR
158 «kJ\(\,\)mol–1» + 4 x 391 «kJ\(\,\)mol–1» / 1722 «kJ»
bonds formed:
E(N≡N) + 2E(H–H)
OR
945 «kJ\(\,\)mol–1» + 2 x 436 «kJ\(\,\)mol–1» / 1817 «kJ»
«ΔH = bonds broken – bonds formed = 1722 – 1817 =» –95 «kJ»
Award [3] for correct final answer.
Award [2 max] for +95 «kJ».
[3 marks]
OR
ΔHvap= −50.6 kJ\(\,\)mol−1 − (−95 kJ\(\,\)mol−1)
«ΔHvap =» +44 «kJ\(\,\)mol−1»
Award [2] for correct final answer.
Award [1 max] for −44 «kJ\(\,\)mol−1».
Award [2] for:
ΔHvap − = 50.6 kJ\(\,\)mol−1 − (−85 kJ\(\,\)mol−1) + = 34 «kJ\(\,\)mol−1».
Award [1 max] for −34 «kJ\(\,\)mol−1».
[2 marks]
total mass of oxygen «= 8.0 x 10–3 g\(\,\)dm–3 x 1000 dm3» = 8.0 «g»
n(O2) «\( = \frac{{8.0{\text{ g}}}}{{32.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \)» 0.25 «mol»
OR
n(N2H4) = n(O2)
«mass of hydrazine = 0.25 mol x 32.06 g\(\,\)mol–1 =» 8.0 «g»
Award [3] for correct final answer.
[3 marks]
«n(N2H4) = n(O2) \( = \frac{{8.0{\text{ g}}}}{{32.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \)» 0.25 «mol»
«volume of nitrogen = 0.25 mol x 24.8 dm3\(\,\)mol–1» = 6.2 «dm3»
Award [1] for correct final answer.
[1 mark]
Examiners report
Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and water.
(i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol phosphine. Calculate the temperature rise using section 1 of the data booklet and the data below.
Standard enthalpy of combustion of phosphine,
Specific heat capacity of air = 1.00Jg−1K−1 = 1.00 kJkg−1K−1
(ii) The oxide formed in the reaction with air contains 43.6 % phosphorus by mass. Determine the empirical formula of the oxide, showing your method.
(iii) The molar mass of the oxide is approximately 285gmol−1. Determine the molecular formula of the oxide.
(i) State the equation for the reaction of this oxide of phosphorus with water.
(ii) Predict how dissolving an oxide of phosphorus would affect the pH and electrical conductivity of water.
pH:
Electrical conductivity:
(iii) Suggest why oxides of phosphorus are not major contributors to acid deposition.
(iv) The levels of sulfur dioxide, a major contributor to acid deposition, can be minimized by either pre-combustion and post-combustion methods. Outline one technique of each method.
Pre-combustion:
Post-combustion:
Markscheme
(i)
temperature rise «\(\frac{{750 \times 1.00}}{{0.2000 \times 1.00}}\)= 3750 «°C/K»
Do not accept −3750.
(ii)
n(P)«=\(\frac{{43.6}}{{30.97}}\)»=1.41«mol»
n(O)«=\(\frac{{100 - 43.6}}{{16.00}}\)»= 3.53«mol»
\(\frac{{n\left( {\rm{O}} \right)}}{{n\left( {\rm{P}} \right)}} = \frac{{3.53}}{{1.41}} = 2.50\) so empirical formula is» P2O5
Accept other methods where the working is shown.
(iii)
\(\frac{{285}}{{141.9}}\)=2.00, so molecular formula=2×P2O5=»P4O10
(i)
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept P4O10 (s) + 2H2O (l) → 4HPO3 (aq) (initial reaction)
Accept P2O5 (s) + 3H2O (l) → 2H3PO4 (aq)
Accept equations for P4O6 /P2O3 if given in a (iii).
Accept any ionized form of the acids as the products.
pH: decreases AND electrical conductivity: increases.
(iii)
phosphorus not commonly found in fuels
OR
no common pathways for phosphorus oxides to enter the air
OR
amount of phosphorus-containing organic matter undergoing anaerobic decomposition is small
Accept “phosphorus oxides are solids so are not easily distributed in the atmosphere”.
Accept “low levels of phosphorus oxide in the air”. Do not accept “H3PO4 is a weak acid”.
(iv)
Pre-combustion:
remove sulfur/S/sulfur containing compounds
Post-combustion:
remove it/SO2 by neutralization/reaction with alkali/base
Accept “lime injection fluidised bed combustion” for either, but not both.
Examiners report
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
\(\begin{array}{*{20}{l}} {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{{\text{(OH)}}}_{\text{2}}}{\text{(aq)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{177.0 kJ}}} \\ {{\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}}&{\Delta {H^\theta } = + {\text{189.2 kJ}}} \\ {{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{{\text{1}}}{{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{285.5 kJ}}} \end{array}\)
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJ\(\,\)kg−1\(\,\)K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
Identify the species responsible for the peak at m/z = 110 in the mass spectrum of hydroquinone.
Identify the highest m/z value in the mass spectrum of quinone.
Markscheme
ΔH = 177.0 – \(\frac{{189.2}}{2}\) –285.5 «kJ»
«ΔH =» –203.1 «kJ»
Accept other methods for correct manipulation of the three equations.
Award [2] for correct final answer.
[2 marks]
203.1 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 57.2 «K»
«Tfinal = (57.2 + 21.8) °C =» 79.0 «°C» / 352.0 «K»
If 200.0 kJ was used:
200.0 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 56.3 «K»
«Tfinal = (56.3 + 21.8) °C =» 78.1 «°C» / 351.1 «K»
Award [2] for correct final answer.
Units, if specified, must be consistent with the value stated.
[2 marks]
C6H4(OH)2+
Accept “molecular ion”.
Do not accept “C6H4(OH)2” (positive charge missing).
[1 mark]
«highest m/z» 108
Only accept exactly 108, not values close to this.
[1 mark]
Examiners report
Magnesium reacts with sulfuric acid:
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
The graph shows the results of an experiment using excess magnesium ribbon and dilute sulfuric acid.
Outline why the rate of the reaction decreases with time.
Sketch, on the same graph, the expected results if the experiment were repeated using powdered magnesium, keeping its mass and all other variables unchanged.
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) \( \rightleftharpoons \) NO(g) + CO2(g) ΔH = –226 kJ
Calculate the activation energy for the reverse reaction.
State the equation for the reaction of NO2 in the atmosphere to produce acid deposition.
Markscheme
concentration of acid decreases
OR
surface area of magnesium decreases
Accept “less frequency/chance/rate/probability/likelihood of collisions”.
Do not accept just “less acid” or “less magnesium”.
Do not accept “concentrations of reagents decrease”.
[1 mark]
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
«Ea(rev) = 226 + 132 =» 358 «kJ»
Do not accept –358.
[1 mark]
2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
OR
2NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Accept ionised forms of the acids.
[1 mark]
Examiners report
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Markscheme
increasing number of protons
OR
increasing nuclear charge
«atomic» radius/size decreases
OR
same number of shells
OR
similar shielding «by inner electrons»
«greater energy needed to overcome increased attraction between nucleus and electrons»
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and
nucleus for M2.
Accept “weaker metallic bonds” for M2.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept “P4O10 (s) + 2H2O (l) → 4HPO3 (aq)” (initial reaction).
«series of» lines
OR
only certain frequencies/wavelengths
convergence at high«er» frequency/energy/short«er» wavelength
M1 and/or M2 may be shown on a diagram.
Mn
Mn (s) + Ni2+ (aq) → Ni (s) + Mn2+ (aq)
wire between electrodes AND labelled salt bridge in contact with both electrolytes
anions to right (salt bridge)
OR
cations to left (salt bridge)
OR
arrow from Mn to Ni (on wire or next to it)
Electrodes can be connected directly or through voltmeter/ammeter/light bulb, but not a battery/power supply.
Accept ions or a specific salt as the label of the salt bridge.
Examiners report
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State the effect of an increase in the total pressure on the equilibrium constant, Kc.
(i) Sketch the potential energy profile for the synthesis of phosgene, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.
Markscheme
(i)
\( \ll {K_{\rm{c}}} = \gg \frac{{\left[ {{\rm{COC}}{{\rm{l}}_{\rm{2}}}} \right]}}{{\left[ {{\rm{CO}}} \right]\left[ {{\rm{C}}{{\rm{l}}_2}} \right]}}\)
(ii)
no effect
(i)
products lower than reactants AND enthalpy of reaction correctly marked and labelled with name or value
activation energy correctly marked and labelled with name or value
Accept other clear ways of indicating energy/ enthalpy changes.
(ii)
lower dotted curve, between same reactants and product levels, labelled “Catalysed”
(iii)
second curve at a higher temperature is correctly drawn (maximum lower and to right of original)
(iv)
greater proportion of molecules have E ≥ Ea or E >Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Do not accept just particles have greater kinetic energy.
Do not accept just “more collisions”.
Examiners report
A student titrated an ethanoic acid solution, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine its concentration.
The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of acid.
Curves X and Y were obtained when a metal carbonate reacted with the same volume of ethanoic acid under two different conditions.
Using the graph, estimate the initial temperature of the solution.
Determine the maximum temperature reached in the experiment by analysing the graph.
Calculate the concentration of ethanoic acid, CH3COOH, in mol dm–3.
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
Explain the shape of curve X in terms of the collision theory.
Suggest one possible reason for the differences between curves X and Y.
Markscheme
21.4 °C
Accept values in the range of 21.2 to 21.6 °C.
29.0 «°C»
Accept range 28.8 to 29.2 °C.
ALTERNATIVE 1
«volume CH3COOH =» 26.0 «cm3»
«[CH3COOH] = 0.995 mol dm–3 \( \times \frac{{50.0\,{\text{cm3}}}}{{26.0\,{\text{cm3}}}} = \)» 1.91 «mol dm−3»
ALTERNATIVE 2
«n(NaOH) =0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
«[CH3COOH] = \(\frac{{0.04975}}{{0.0260}}\) dm3 =» 1.91 «mol dm–3»
Accept values of volume in range 25.5 to 26.5 cm3.
Award [2] for correct final answer.
«total volume = 50.0 + 26.0 =» 76.0 cm3 AND «temperature change 29.0 – 21.4 =» 7.6 «°C»
«q = 0.0760 kg x 4.18 kJ kg–1 K–1 x 7.6 K =» 2.4 «kJ»
Award [2] for correct final answer.
«n(NaOH) = 0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
OR
«n(CH3COOH) = 1.91 mol dm–3 x 0.0260 dm3 =» 0.04966 «mol»
«ΔH = \( - \frac{{2.4\,{\text{kJ}}}}{{0.04975\,{\text{mol}}}} = \)» –48 / –49 «kJ mol–1»
Award [2] for correct final answer.
Negative sign is required for M2.
«initially steep because» greatest concentration/number of particles at start
OR
«slope decreases because» concentration/number of particles decreases
volume produced per unit of time depends on frequency of collisions
OR
rate depends on frequency of collisions
mass/amount/concentration of metal carbonate more in X
OR
concentration/amount of CH3COOH more in X